The pressure of a gas is increased from 1.5 atm to 3.0 atm. What will the new volume of the gas be if the original volume was 4.5 L?
2.3 atm (must have units!)
At standard pressure, as temperature increases, the volume of a latex balloon __________ because there is a(n) ____________ relationship between temperature and volume.
increases; direct
What is shown in the diagram marked "A"
Provide the NAME and DESCRIPTION
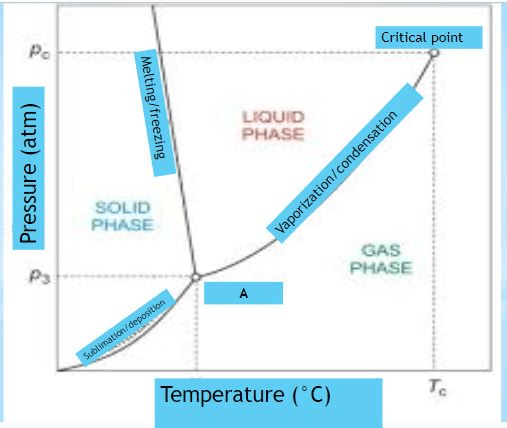
triple point:
The pressure and temperature at which all three states exist at the same time.
What is occurring at #4 in the image?
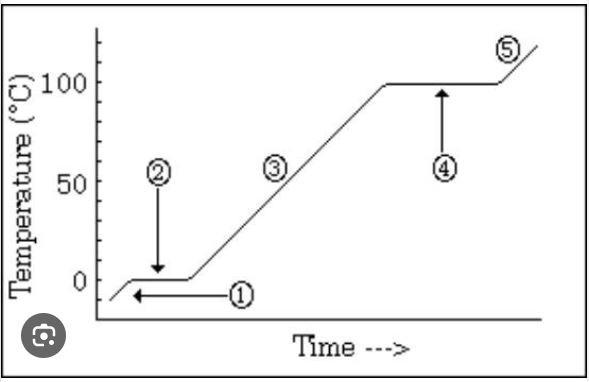
vaporization BEST ANSWER
A graphic way to show the effect of temperature changes due to energy changes
Heating/cooling or heating curve (on vocab list as heating curve)
The initial air pressure in a submarine is 2.3 atmospheres and after rising to the surface of the ocean is 1.0 atm. What is the temperature inside the sub initially if when it rises to the surface its temperature ends up being 38 ℃?
7.0 x 102 K
(must be in sci. not. because of the 2 s.f. requirement.)
A football inflated inside then taken outside on a cold day shrinks slightly. Which law is described?
Charles' Law
Identify what is marked "A" on the diagram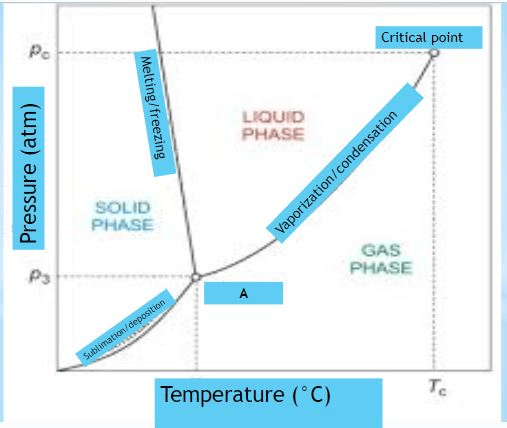
triple point
Which number is marking the state of matter with the MOST density?
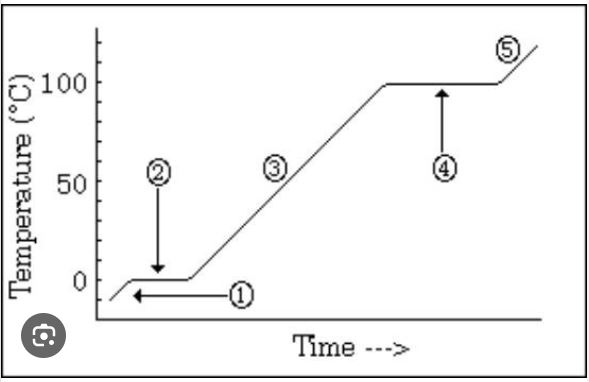
#1
a measure of the average kinetic energy of the particles in an object
temperature
What pressure is exerted by 0.625 mol of a gas in a 45.5 L container at a temperature of 25.0 ℃?
0.340 atm (must have units and 3 s.f.)
Deep sea fish die when brought to the surface.
This is due to the pressure decreasing as they come to the surface, so the volume of gases in their body increases, causing their cells, swim bladder and other membranes to pop.
Which law is described?
Boyle
At normal pressure, what is the boiling point for the substance shown in the phase diagram?
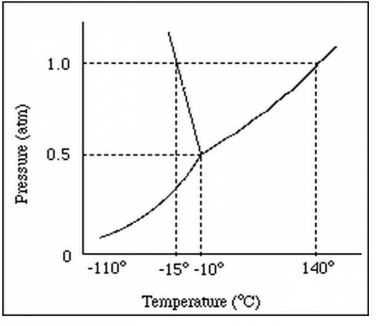
-15 degrees C (MUST HAVE UNITS!)
Which number(s) mark the area on the image where average kinetic energy remains the SAME?
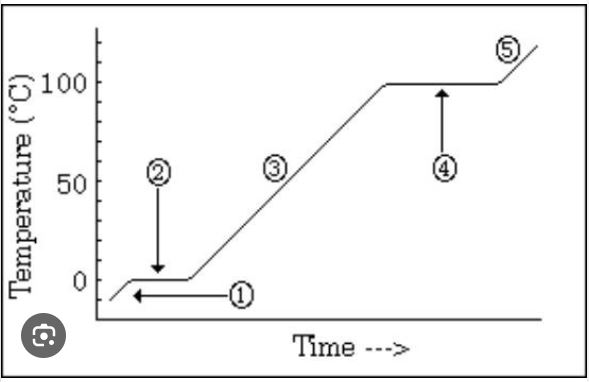
#2 and #4
1 atm and 0 degrees Celsius
STP or standard temperature and pressure
A gas at 305 K occupies a volume of 6.50 liters. What will its volume be at 255K?
5.43 L (must be in 3 s.f and have units)
The CO2 cylinder of an air soft gun turns cold when the pressure is released.
Which law is described?
Gay-Lussac
At normal pressure, what is the boiling point for the substance shown in the phase diagram?
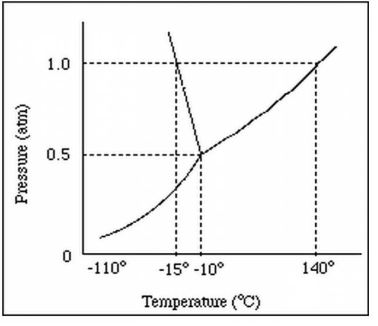
140 degrees C (MUST HAVE UNITS!)
What numbers on the image are labeling areas where the average kinetic energy is INCREASING (adding heat) or DECREASING (heat loss)
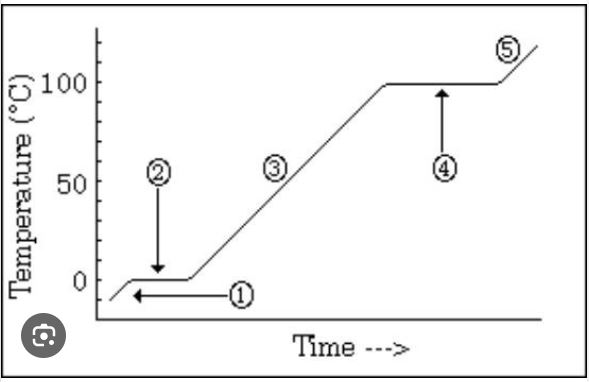
#1, #3, #5
A graph of pressure versus temperature that shows the
conditions under which the phases of a substance exist
phase change diagram OR phase diagram
A sample of gas occupies a volume of 1.2L at a pressure of 0.75 atm and a temperature of 22oC. What will the volume be at a pressure of 1.25 atm and a temperature of 70oC?
0.84 or .84 L
(Must have units and 2 s.f.)
A balloon placed in the freezer will shrink in volume.
Which law is described?
Charles'
What phase change occurs at 30 atm as the temperature increases from 0 degrees celsius to 175 degrees celsius?
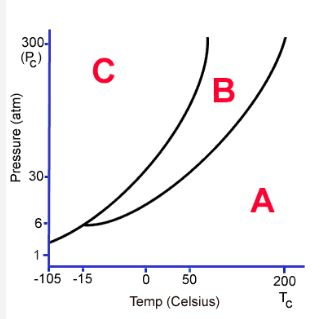
vaporization ONLY (it's the best word for it!)
How much energy does it take to heat the substance in the diagram from 30 degrees C to 60 degrees C?
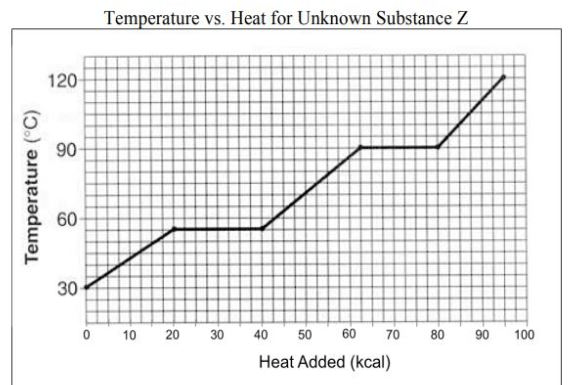
20 kcal (MUST HAVE UNITS)
the temperature and pressure conditions at which the solid, liquid, and gaseous phase of a substance coexist at equilibrium.
triple point