Which of the following contains ONLY covalent bonds?
A) CuCO3
B) KC2H3O2
C) NaCl
D)IBr
E) None of these
F) All of these
D
Which of the following has an expanded octet?
A) H2O
B) PCl5
C) KCl
D) RbNO3
E) None of these
F) All of these
B
What is the hybridization of carbon in methane?
What is the molecular geometry and hybridization of XeF4?
Square planar sp3d2
Which of the following combinations are not possible for quantum numbers?
n=1, l=0, ml=0, ms=1/2
n=2, l=1, ml=-1, ms=-1/2
n=3, l=3, ml=3, ms=1/2
n=4, l=3, ml=-2, ms=-1/2
n=3, l=3, ml=3, ms=1/2
What type of electromagnetic radiation has the longest wavelength?
Bonus: What is the generally accepted range (in nm)?
Radio waves!
Greater than 108 nm
Set these molecules in order of increasing dipole moment.
BF2Cl, BCl3, BF2I
BCl3 < BF2Cl < BF2I
Draw the Lewis structure of NH2CH2COOH and tell me how many lone pairs of electrons are found on this molecule?
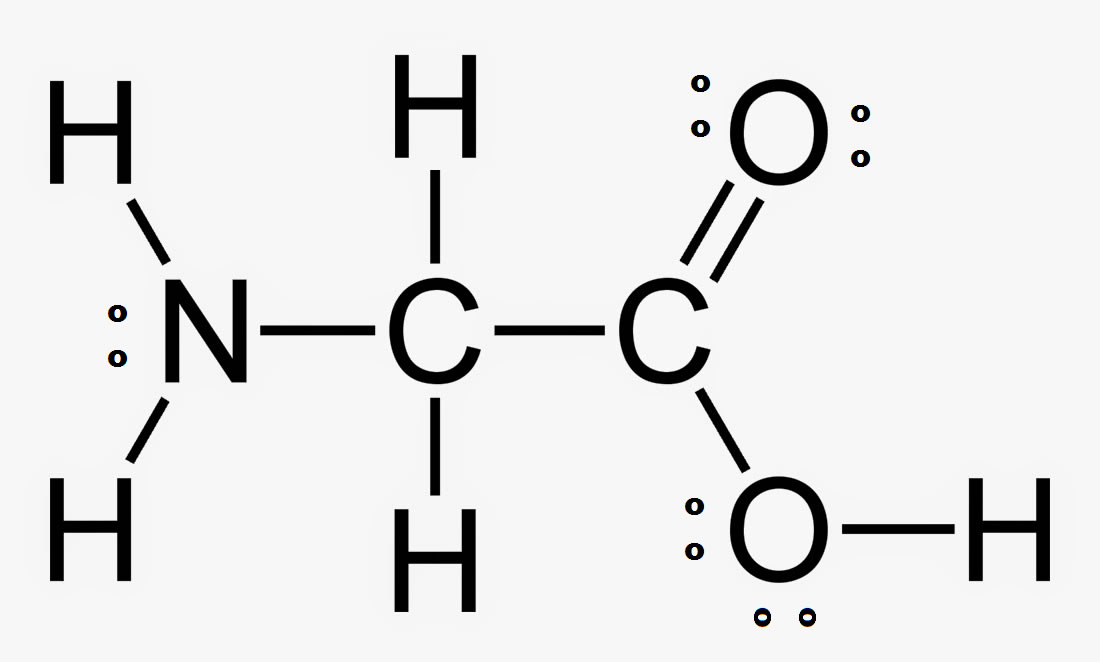
5 lone pairs
What is the hybridization of the oxygen in H2O?
sp3
Given the rxn below and the given bond energies below, what is the deltaH of the rxn? Also for a bonus of 100 points, is this reaction favorable in the forward direction?
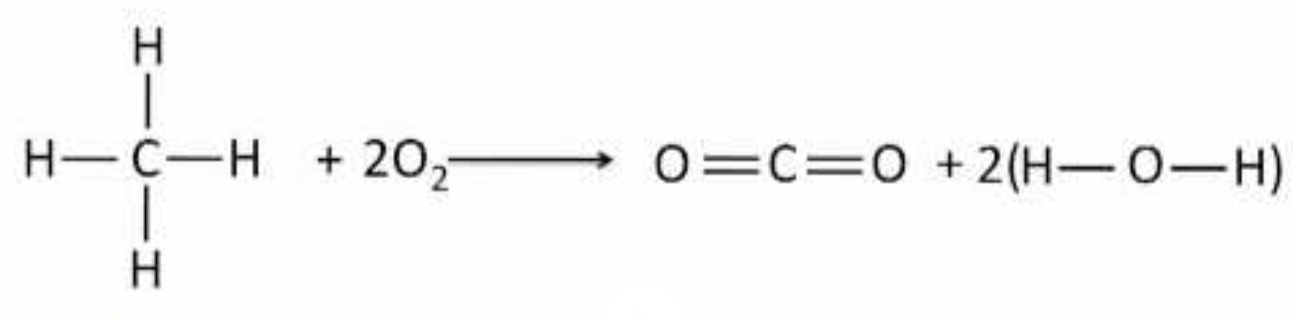
C-H 413 kJ/mol
C=O 799 kJ/mol
O-H 463 kJ/mol
O2 495 kJ/mol
deltaH = bonds broken - bonds formed
[2(O2) + 4(C-H)] - [2(C=O) + 2(2(O-H))]
[2(495) + 4(413)] - [2(799) + 4(463)]
2642 - 3450 = -808 kJ/mol
Given the following emission transition n=5 -> n=2 what type of wave would be emitted?
Blue light or just visible light!
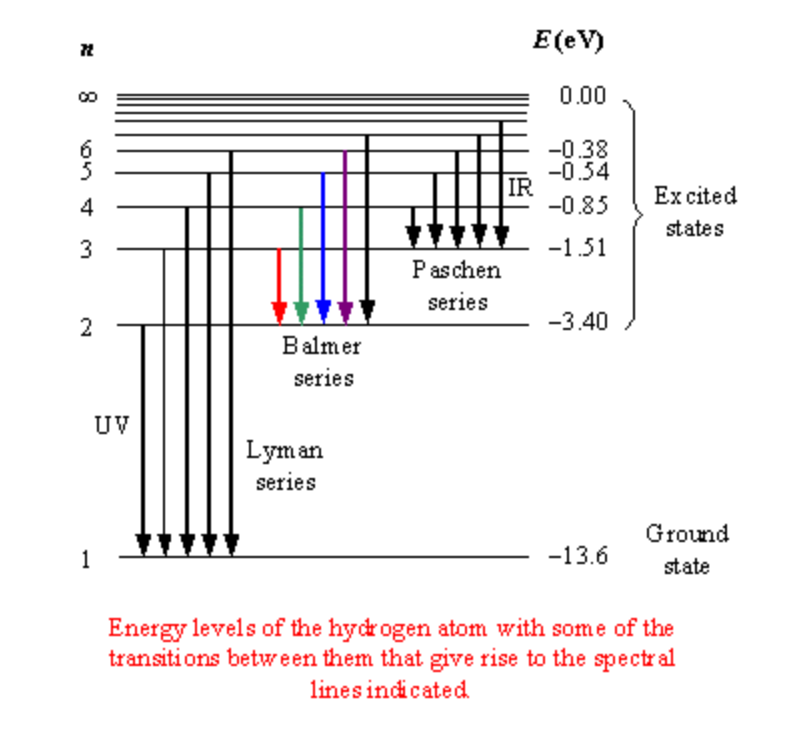
Calculate the wavelength(nm) of a photon that has an energy of 4.2x10-19 J.
E = hν; λ = c/ν; h = 6.626x10-34 J*s; c = 3.00 x108 m/s
λ=ch/E => 4.7E-7 m => 470 nm
Order the bonds in order of increasing dipole moment.
H-Cl, H-F, H-I, H-Br
H-I < H-Br < H-Cl < H-F
Which of the following have two or more equivalent resonance structures?
CH2N2, N2O, COOHCH3, HNO3, OCN
HNO3
What types of hybridization does nitrogen mostly occur as?
sp, sp2, sp3
What is the geometry of XeF4+2?
Seesaw
How many valence electrons are in Iron?
8
[Ar] 4s23d6
Which has the largest radius?
Fe, Cu, Mn, B, Te
Manganese!
Compare CH4 and CF4
Draw their Lewis structures.
What are their hybridizations and geometries?
Is the C-F or the C-H bond more polar? Which one is stronger?
hybridizations: sp3
geometries: tetrahedral
more polar bond: C-F
stronger bond: C-F
Draw the Lewis structure for CO3-2
What is the formal charge of each atom?
C : 0
OA: -1
OB: -1
OC: 0
Which is not paired correctly?
CF4 : sp3
HNO3: sp2
PF5: sp3d2
FeCl3: sp2
PF5: sp3d
Which of the following bonds is the strongest?
Si-H; Si-Cl; H-Cl; O-H; Si=N, N=C
Si=N
What is the electron configuration of Iron?
[Ar] 4s23d6
1s2 2s2 2p6 3s2 3p6 3d6 4s2
What has the largest atomic radius on the whole periodic table?
Fr, Francium
How many pi bonds and sigma bonds are present?

6 pi bonds
43 sigma bonds
How many bonding pairs of electrons are found around the chlorine atom in the perchlorate ion?
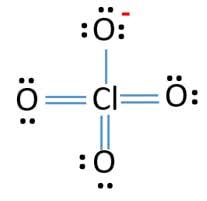
7
What is the hybridization of SeF4-2?
sp3d2
Which has the smallest bond angles?
PCl5, SF6, XeF4, SF4
SF4
Show me an example of an Aufbau principle violation.
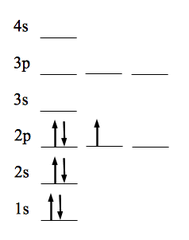
This is an example!
Put these in order of increasing electron affinity.
Sc, V, Ti, Cr
Ti < Sc < V < Cr
Given XeF4, tell me the following:
How many pi bonds?
How many sigma bonds?
How many lone pairs are found on Xe?
What is the hybridization?
What is the geometry?
pi bonds: 0
sigma bonds: 4
lone pairs on Xe: 2
hybridization: sp3d2
geometry: square planar
If you know the bond energy for O2 and N2 and find the bond energy for NO, what would this energy be in relation to the given bond energy?
A) Sum of the two values given
B) Difference of the two values given
C) Average of the two values given
D) Product of the two values given
Which of the following is diamagnetic?
Li, W, Cs, Hg, Gd
Hg
Put these in order of increasing 1st ionization energy.
Mn, Mo, Fe, Rh
Mo < Mn < Rh < Fe