Describe collision theory and transition state theory
Collision theory- a model that explains rxn rate as based on the number of solute particles
-Molecules must “collide” to react
• This is the basis of the rate law rate = k[A]m[B]n
• Increase conc of reactant increase # of possible collisions
• Not all collisions result in a reaction (most do not result in a rxn)
Transition state theory- a model that explains how the energy of reactant collisions is used to form a
high-energy transitional species that can change to reactant to product
- Involves a transition state or activated species
• A high energy, unstable species
In a closed container, vapour pressure of a liquid depends upon
- Volume of the container
- Temperature
- Volume of the liquid
- All of the above
Temperature
Explanation: The tendency of a material to change into a gaseous state is referred to as vapour pressure. In other words, the pressure exerted by a liquid’s vapour in thermodynamic equilibrium with the condensed phases in a closed system is termed as vapour pressure
Suppose you are studying a reaction, and you have the following information:
- The rate constant (k) for the reaction at 300 K is 0.02 s⁻¹.
- The activation energy (Ea) for the reaction is 40,000 J/mol.
Calculate the pre-exponential factor (A) for this reaction.
2.16 x 10^-9
Explain why the rate of disappearance of NO and the rate of formation of N2 are not the same in the reaction, 2CO(g) + 2NO(g) → 2CO2(g) + N2(g)
Because of the 2:1 stoichiometric ratio between NO and N2, the NO must use 2 moles for each mole of N2 produced. This means that the rate of consumption of NO is twice as fast as the rate of production of N2.
Why is the instantaneous rate of reaction preferred over the average rate? Or why is the rate of the reaction not uniform throughout?
This is because the rate of any reaction depends upon the reactant’s concentration at the given time. As the concentration of each reactant decreases with time, the rate of reaction also keeps changing.
Describe the 4 factors that can be used to manipulate rate
conc
physical state
temp
the use of a catalyst
Which of the following has equal boiling point?
- 0.1 M Na2SO3
- 0,1 M C12H22O11
- 0.1 MgCl2
- 0.1 M Al(NO3)3
Explanation: Boiling point will be the same for those solutes which will have the same value for van’t Hoff factor.
Na2So3: 3
C12H22O11: 1 (nonelectrolyte)
Al(NO3)3 : 4
MgCl2: 3
Hence, Na2SO3 and MgCl2 will have the same value for van’t hoff factor(i).
A first-order chemical reaction has a rate constant (kk) of 0.02 s⁻¹. Calculate the following:
- The time it takes for the initial concentration of the reactant to decrease to half of its original value.
- The concentration of the reactant after 30 seconds if the initial concentration is 0.1 M.
1. 34.65s
2. 0.05M
For the reaction, A → products, a graph of [A] versus time is a curve. What can be concluded about the order of this reaction? It can't be a zero, first, or second order rxn?
It cannot be a zero order rxn since the graph is a curve.
The threshold energy is equal to
- Average kinetic energy of molecules of reactants
- Normal energy of reactants – activation energy
- Activation energy + normal energy of reactants
- Activation energy – normal energy of reactants
(3)
Explanation: The activation energy is the excess energy that is acquired by the molecules in order to achieve the threshold energy.
What is a catalyst
A chemical agent that speeds up a rxn
Which of the following aqueous solutions has the highest vapour pressure at 300 K?
- 1 M Na3PO4
- 1 M CaCl2
- 1 M KNO3
- 1 M C6H12O6
1 M C6H12O6
The Van’t Hoff Factor(i) quantifies the effect of a solute on various colligative properties of solutions.An electrolytic solute’s van’t Hoff factor is always equal to the number of ions in which it is ionised. It is equal to one for a non-electrolytic solute.
Upon ionisation of electrolytic solutes Na3PO4, CaCl2 and KNO3, the value of the van’t hoff factor(i) comes out to be 4,3 and 2 respectively. Because C6H12O is a non-electrolytic solute, it has i=1.
The greater the value of the Van’t Hoff factor(i), the lower the vapour pressure, and vice versa. As a result of the lower van’t hoff factor(i) value, C6H12O6 has a higher vapour pressure.
The vapor pressure of substance X at its normal boiling point (100 degrees celsius) is 500 mm Hg. The molar enthalpy of vaporization (ΔHvap) is 35 kJ/mol. Substance Y has a normal boiling point of 80°C. Calculate the vapor pressure of substance Y at its boiling point.
263.8 mmHg
In the reaction H2O2(aq) → H2O(l) + ½ O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10–4 M s–1. What will be [H2O2] at t = 35 s?
Use the definition of rate (change in concentration over change in time), recognizing that the initial rate for the loss of hydrogen peroxide is negative and at t = 0 s, [H2O2] = 0.2546 M.
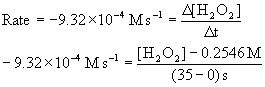
[H2O2] = 0.2220 M
The influence of temperature on the rate of the reaction is determined by
- Arrhenius equation
- Nernst’s equation
- Gibbs-Helmholtz equation
- Van’t Hoff equation
(1)
Explanation: The Arrhenius equation has a direct relation between temperature and the rate constant.
How does T affect Rate?
Higher T- Larger K- Increased rate
Examine the diagram below and select the appropriate options.

- There will be no substance movement across the membrane.
- CaCl2 will flow towards the KCl solution
- The CaCl2 solution will be approached by KCl.
- The osmotic pressure of 0.1 M KCl solution is greater than that of 0.05 M CaCl2 solution.(assuming complete dissociation of electrolyte)
Answer: d) The osmotic pressure of 0.1 M KCl solution is greater than that of 0.05 M CaCl2 solution.
Explanation: The diagram depicts the osmosis phenomenon. Osmosis is the flow of solvent molecules from its high concentration to its low concentration.
Only solvent molecules, not solute particles, move across the membrane. Because CaCl2 and KCl are solutes, they will not move or flow.
Osmotic pressure,𝝅 = i×c×R×T
Osmotic pressure is directly proportional to the magnitude of (i×c) provided temperature and R are constant.
Value of “i” is 2 and 3 for KCl and CaCl2 respectively.
For KCl, 𝝅 = 2×0.1= 0.2
For CaCl2,𝝅 = 3 ×0.05=0.15
We can clearly see, 0.1 M KCl has higher osmotic pressure than 0.05 M CaCl2 .
You have three solutions: Solution X, Solution Y, and Solution Z.
- Solution X contains 0.2 moles of potassium chloride (KCl) dissolved in 200 mL of water.
- Solution Y contains 0.1 moles of sodium chloride (NaCl) dissolved in 200 mL of water.
- Solution Z contains 0.3 moles of glucose (molar mass = 180.16 g/mol) dissolved in 200 mL of water.
- all have a H2O vapor pressure of: 0.0361
Calculate the following for each solution:
- The vapor pressure lowering (Raoult's law) at the given temperature.
x) 6.5 x 10^-4
y)3.21 x 10^-4
z) 9.49 x 10^-4
The rate of the following reaction in aqueous solution is monitored by measuring the number of moles of Hg2Cl2 that precipitate per liter per minute. The data obtained are listed in the table.
2 HgCl2(aq) + C2O42–(aq) → 2 Cl–(aq) + 2 CO2(g) + Hg2Cl2(s)
Experiment[HgCl2] (M)[C2O42–] (M)Initial rate (mol L–1 min–1)
10.1050.151.8×10–5
20.1050.151.8×10–5
30.0520.307.1×10–5
40.0520.158.9×10–6
(a) Determine the order of reaction with respect to HgCl2, with respect to C2O42– and overall.
(b) What is the value of the rate constant k?
(c) What would be the initial rate of reaction if [HgCl2] = 0.094 M and [C2O42–] = 0.19 M?
Use the method of initial rates to find the orders of reaction in each component. This will allow evaluation of the rate constant and the initial rate of reaction at any other condition.
(a) Rate = k[HgCl2]m[C2O42–]n
Compare the rates in experiments 1 and 2 (or 3 and 4) to find the order in oxalate ion:
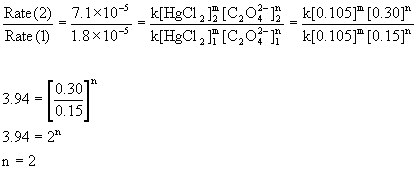
Compare the rates in experiments 2 and 3 (or 1 and 4) to find the order in mercury(II) chloride:
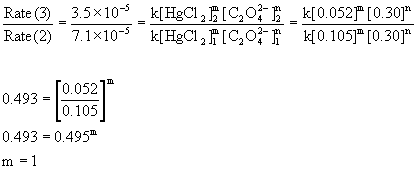
Therefore, the reaction is first order with respect to mercury(II) chloride and second order with respect to oxalate. The overall order is the sum of these, 2 + 1 = 3, third order.
(b) To find the rate constant, use the rate equation and solve for k:
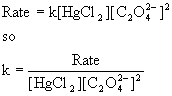
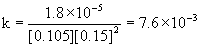
The average k = 7.6×10–3 M–2min–1
(c) Rate = k[HgCl2][C2O42–]2
Rate = (7.6×10–3 M–2min–1)[0.094 M][0.19 M]2 = 2.6×10–5 M min–1
The experimentally determined rate expression for the reaction: NO2(g) + CO(g) → CO2(g) + NO(g) is Rate = k[NO2]2. Suggest the mechanism for the reaction.
As the rate depends upon the decomposition of NO2,the possible mechanism can be:
Step 1: NO2 + NO2 → NO + NO3 (slow)
Step 2: NO3 + CO → CO2 + NO2 (fast)
What is molecularity?
the number of reactant particles in an elementary reaction
When 0.643 g of a compound is added to 50 mL of benzene (density:0.879g/mL),the freezing point is reduced from 5.51 to 5.03 degrees Celsius. What is the compound’s molecular mass?(Kf for benzene = 5.12)
156 g/mol
Let molecular mass of solute(compound) be y
Density of benzene = Mass of benzene/Volume
0.879 g/mL= Mass of benzene/ 50mL
Mass of benzene = 43.95 g = 0.04395 Kg
Moles of solute( compound) = Weight of solute/ Molecular mass of solute
= 0.643 g/ y
Molality(m) = Moles of solute/ Mass of benzene( Kg)
= 0.643 g/(y × 0.04395 Kg)
Depression in freezing point, ΔTf= Kf× m
(5.51-5.03) K = 5.12 K Kg/mol ×[ 0.643 g/(y × 0.04395 Kg)]
On solving, y = 156 g/mol
You have two solutions: Solution A and Solution B.
Solution A contains 0.2 moles of glucose (molar mass = 180.16 g/mol) dissolved in 500 mL of water.
Solution B contains 0.1 moles of sucrose (molar mass = 342.3 g/mol) dissolved in 500 mL of water.
Calculate the following for both solutions:
- The molality of each solution.
- The boiling point elevation of each solution, assuming the boiling point constant (Kb) for water is 0.512°C kg/mol.
1. A= 0.4m
B= 0.2m
2. A= 0.2048 degrees celsius
B=0.1024 degrees celsius
The following is proposed as a plausible reaction mechanism:
A + B → I(slow)
I + B → C + D(fast)
What is (a) the net reaction described by this mechanism and (b) a plausible rate law for the reaction?
(a) To find the net reaction, sum up all the reactions in the mechanism and eliminate common species on each side of the equation:
A + B + I + B → I + C + D
net: A + 2B → C + D
(b) The rate law is determined by the slow step in the reaction mechanism. Since a reaction mechanism is composed of elementary reactions, the rate law for the slow step can be written by inspection, with the orders of reaction being the stoichiometric coefficients:
Rate = k[A][B]
Verify the statement: Slow reactions require less energy of activation as compared to the fast reactions. Is this statement right or wrong? Why?
This statement is wrong as the energy of activation is inversely proportional to the rate of the reaction.