Is a rotting banana is a physical or chemical change?
Chemical
What part of the scientific method is this? If I add salt to the pancakes, then they will taste better.
Hypothesis
What type of data is defined as the following: information that cannot be counted, measured or easily expressed using numbers.
qualitative
Does the symbol "Br2" represent an element, compound, or molecule of an element?
Molecule of an element
A student measured the mass of some sodium chloride for a lab. The lab called for 6.25 g (the accurate measurement), but the student measured 6.00 g instead. What is the percent error for this mass?
4%
How can sand and iron filings be separated?
By a magnet
Make an observation and inference about the following photo
Answer varies
A conclusion based on observations that may or may not be correct
Inference
What is the chemical symbol for Radon?
Rn
Adriana determined the density of calcium to be 2.38 g/cm3 . Based on the actual density found on Table S, what is her percent error?
83.76 %
What is the diagram showing the mixture of?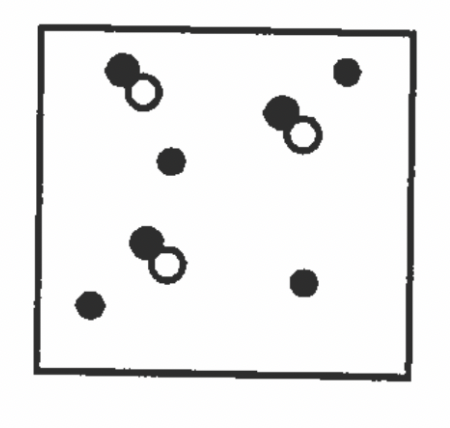
An element and compound
What is a chemical change?
A chemical change is that results in the formation of a new substance
Is the following quantitative or qualitative data:
Most of my textbooks have over 240 pages.
quantitative
2 or more atoms of different elements that are chemically combined
Compounds
Which particle diagram represents one pure substance only?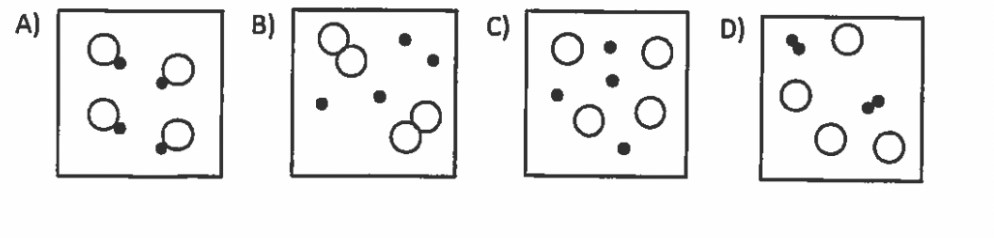
A
The density of Cobalt is 8.86 g/cm3. What is the volume if the mass is 2.73g?
.31 cm3
What type of mixture is blood and why? You must support your answer
Homogeneous. You can't see the red/white blood cells, platelets, salt, water, etc.
Is evaporation a physical or chemical change and why
Physical because it is a change in the state of matter. No new substance is formed
What part of the scientific method summarizes the purpose of the experiment, hypothesis, and results
Conclusion
How close a measurement is to the true or accepted value
Accuracy
Is sulfuric acid (H2SO4) an element or compound? You must support your answer
Compound- Contains hydrogen, sulfur, oxygen
Some students were measuring the volume of a cube shaped wooden block. They measured the length to be 5.08 cm, the width to be 5.09 cm, and the height to be 5.10 cm. The actual volume of the block was 15.3 cm3. What was their percent error?
761.9%
A mixture of water, chocolate powder, baking soda, oil, and wood chips is filtered. What substance(s) is left in the filter paper and why?
Wood chips because it does not dissolve in water
Provide 3 pieces of evidence that a chemical change occurred
Gas Forms
Temperature Change
Odor
Color Change
Precipitate Forms
Light Forms
What is the dependent and independent variable in the question?
If I raise the temperature, then mold will grow faster.
D- How long it takes for mold to grow
I- Temp
The base unit for volume is ________
Grams
Identify 3 of the diatomic elements
HOFBrINCl
Hydrogen, Oxygen, Fluorine, Bromine, Iodine, Nitrogen, Chlorine
In an experiment to determine density, students were measuring mass and volume. They found the mass of their substance to be 8.12 g and the volume to be 1.9 cm3. The actual density was 4.5 g/cm3. What was their percent error?
5.1%