Avagadro's Number
6.023*10^23
Number of electrons on a Carbon atom
Br-
Bromide
The missing Product in the following reaction:
NaOH(aq)+HCl(aq)->NaCl(aq)+___
H2O
An atom with 11 protons, 12 Nertrons, 10 Electrons
1123Na+, Sodium-23 cation
Relationship betwen bar and mmHg
1bar=750mmHg
Number of sigma and pi bonds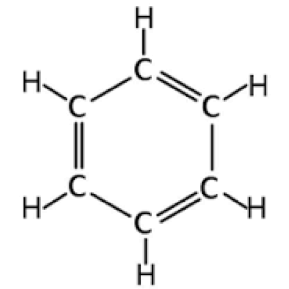
12 sigma, 3 pi
LiF
Lithium Fluoride
Balanced reaction for:
Al(s)+O2(g)->Al2O3
4Al(s)+3O2->2Al2O3
pH of a 0.047M HCl solution
1.32
Grams of carbon in 4.2mol
50.4g
The most electro negitive (B,O,N,C)
O
OH-
Hydroxide
The limiting reagent when 3molH2 react with 4molO2 to form H2O
H2
The Ideal Gas Law
PV=nRT
Moles of H3PO4 in a 5.34L, 0.0124M solution
0.066mol H3PO4
Number of lone pairs on NH3
HCl
Hydrochloric acid
Net Ionic Equation for:
NaCl(aq)+AgNO3(aq)->AgCl(s)+NaNO3(aq)
Ag+(aq)+Cl-(aq)->AgCl(s)
The reigions of the elecromagnrtic specrum with higher energy thal visable light
Ultraviolet,X-ray,Gamma-ray
Number of H atoms in 3.14mg H2SO4
3.85*10^23
Formaldehyde has a formula of CH2O draw the structure

CCl4
Carbon tetrachloride
The pH of a solution containig 0.00125mol NaOH, and 0.00250mol HCl.
2.6
The sign for delta H in an exothermic reaction
Negitive