Which intermolecular force is the weakest?
London Dispersion Forces
How many atoms are in one face centered cubic cell?
4 atoms
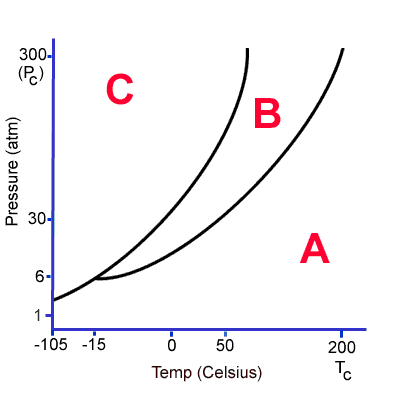 Point A represent solid
Point A represent solid
What is the formula for molarity and the formula for molality including the right symbols!


Which intermolecular force is present in a bucket of this molecule: PCl3 ?
Dipole-Dipole
this is a polar molecule.
There is one atom in one unit of this type of cell. What type of cell is this?
Simple cubic cell
Increasing the temperature increases the solubility of gasses and liquids in water.
False. Increasing the temperature increases the solubility of solids and liquids and decreases the solubility of gasses.
If 0.100 mole of NaCl is dissolved into 100.0 grams of pure H2O. What is the mole fraction of NaCl?
.018
100.0 g / 18.0 g /mol = 5.56 mol of H2O
Add that to the 0.100 mol of NaCl = 5.56 + 0.100 = 5.66 mol total
Mole fraction of NaCl = 0.100 mol / 5.66 mol = 0.018
Indicate the strongest intermolecular force between these two molecules: HCl and NH3
Hydrogen bonding
H atoms can bond to electronegative N, O, F
What is the density of a unit cell with if the mass of the unit cell is 104.5 grams and the edge length of the unit cell is 6 cm.
0.484 g/cm3
104.5/(63)= 0.484 g/cm3
H2 Has a higher boiling point than I2.
false. both have dispersion forces so the smaller element by molar mass hydrogen has a smaller mass then iodine and so less polarizable and weakest force so I2 has higher boiling point.
Rubies and sapphires are gemstones where their crystal structure is mostly comprised of aluminum oxide, Al2O3. Find the parts by (%) mass of aluminum and oxygen.
mass Al = 26.98 g/mol
mass O = 16.00 g/mol
There are two atoms of aluminum in a Al2O3 molecule, so
massAl = 2⋅26.98 g/mol = 53.96 g/mol
There are three atoms of oxygen:
massO = 3⋅16.00 g/mol = 48.00 g/mol
Add these together to get the total mass of Al2O3
massTOTAL = massAl + massO
massTOTAL = 53.96 g/mol + 48.00 g/mol
massTOTAL = 101.96 g/mol
mass % Al = (53.96 g/mol /101.96 g/mol) x 100
mass % Al = 0.53 ⋅ 100
mass % Al = 53%
![]()
mass % O = 0.47 ⋅ 100
mass % O = 47%
Which Molecule will have a higher vapor pressure?
HCl or NH3
HCl
intermolecular force is weaker (dipole-dipole vs hydrogen bonds)
What is the density of Ag in a face centered cubic unit if the edge length of the unit cell is 408.7 x 10-10 cm and the atomic mass of silver is 108 g/mol.
10.51 g/cm3
Density= (number of atoms in type of unit cell x atomic mass)/ (edge length3 x avagadro's number)
4 x 108 =432
(408.7 x 10-10 cm)3 x 6.022 x 1023 = 41.11
432/41.11 = 10.51
Dipole-dipole is the strongest intermolecular force.
False. the strongest intermolecular force is ion dipole.
An aqueous acetic acid solution with .839 M is prepared with a total volume of .50 L. The molecular weight of acetic acid is 60.05 g/mol. What mass of acetic acid is necessary to create this solution?
25.22 grams
molar concentration = moles solute/ L solution
mole solute = .839 M x .500 L = .420 mol
.420 mol x 60.05 g/mol = 25.22 grams
Rank these molecules from lowest to highest boiling point: C2H4, CH4, Ne, H3COCH3.
Ne<CH4<C2H4<H3COCH3
The stronger the intermolecular force the higher the boiling point
Aluminum forms a face centered cubic unit cell and has a density 2.70 g/cm3 and molecular weight 26.98 gram/mol. Determine the Edge length of the unit cell in cm.
4.049 x 10-8 cm.
4 atoms in face centered and one atom is 26.98 g/mol
4x26.98=107.92
107.92/(6.022 x 1023)= 1.79E-22 (g/unit cell)
V = M/D
1.79E-22/2.7=4.049 x 10-8 cm
CH3CH2CH2CH2CH2CH3 is less soluble in water than
CH3OH.
true! like dissolve like polar vs. non polar.
A 4 g sugar cube (Sucrose: C12H22O11) is dissolved in a 350 ml teacup of 80 °C water. What is the molality of the sugar solution? The Density of water at 80° = 0.975 g/ml and sucrose molecular weight is 342 g/mol.
molality = moles solute / kg solvent
find mole in solute 4 g/ 342 g/mol = .0117 mol
density = mass/volume
mass = density x volume
mass = 0.975 g/ml x 350 ml
mass = 341.25 g
mass = 0.341 kg
molality = 0.0117 mol / 0.341 kg
molality = 0.034 mol/kg