What do we call anything that has mass and takes up space? (p.98)
Matter
What are invisible, negatively charged particles? (p.102)
Electrons
What do we call matter that is made of only one kind of atom? (p.106)
An element
What category of elements generally have a shiny luster, and are good conductors of heat and electricity? They are ductile (can be drawn into wires without breaking), and malleable (can be bent or pounded into various shapes). (p.110)
Metals
Matter that has the same composition and properties throughout is called what? (p.113)
Substance.
Elements, such as a bar of gold or a sheet of aluminum, are substances. When different elements combine, other substances are formed.
What is a small particle that makes up most matter? It comes from the Greek word meaning "cannot be divided."
An atom
What is the positively charged, central part of an atom? (p.103)
Nucleus
What do we call the chart that organizes and displays the elements? (p.107)
The Periodic Table of Elements
Nonmetals
What do you call a substance whose smallest unit is made up of atoms of more than one element bonded together? (p.113)
Compound.
Compounds have properties that are different from the elements that make them up. Water is distinctly different than the elements that make it up.
Compounds are made of a specific ratio of elements. They have a formula that tells how many of each type of atom are present.

Give an example of something that is NOT matter. (p.98)
Light, heat, emotions, thoughts, ideas, etc.
What are the positively charged particles found in the nucleus of an atom? (p.103)
Protons
What is the top number in an element's block? It tells the number of protons in the nucleus of each atom of that element. (p.109)
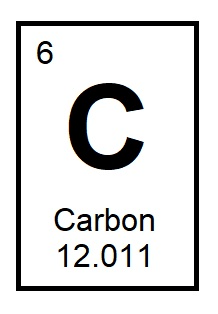
Atomic Number.
The atomic number tells the number of protons in the nucleus of each atom of an element.
In an uncharged atom (an atom that is neither positive nor negatively charged), the number of electrons is exactly the same as the number of protons.
Which category of elements have characteristics of metals and nonmetals? They are solids at room temperature. Some are shiny, and many are conductors, but not as good at conducting heat and electricity as metals. (p.111)
Metalloids
What do we call it when two or more substances (elements or compounds) come together, but do not combine to make a new substance? (p.115)
Mixture.
Unlike compounds, the proportions of the substances in a mixture can be changed without changing the identity of the mixture. For example, if you put sand and water in a bucket, you have a mixture of sand and water. Adding more water or sand will not change this. It is still a mixture of sand and water.
TRUE OR FALSE?
If an orange were as large as the Earth, each of its atoms would be marble-sized. (p.101)
Believe it or not, this is absolutely TRUE!
Atoms are so small, it would take about 1 million of them lined up in a row to equal the thickness of a human hair.
What are the uncharged particles in a nucleus of an atom called? (p.104)
Neutrons
What do we call the number found below the element symbol on the periodic table? It is the weighted average mass of the isotopes of an element. (p.110)
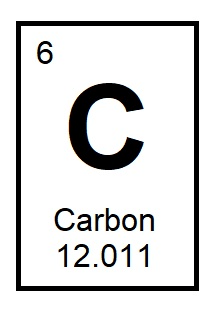
Which category of elements makes up most of the periodic table? (p.110)
Metals
What do we call a mixture that is the same throughout? No matter how hard you look, you cannot see the individual parts that make up the mixture. (p.117)
Homogeneous mixtures
What scientific idea says that matter is not created or destroyed -- it only changes form? (p.100)
The law of conservation of matter
In early atom models, electrons were placed in fixed orbits around the nucleus (similar to how planets orbit the sun).
The current model shows that the electrons have characteristics similar to waves, and that they don't have defined orbits. It is believed that electrons move in a way referred to as what? (p.104-105)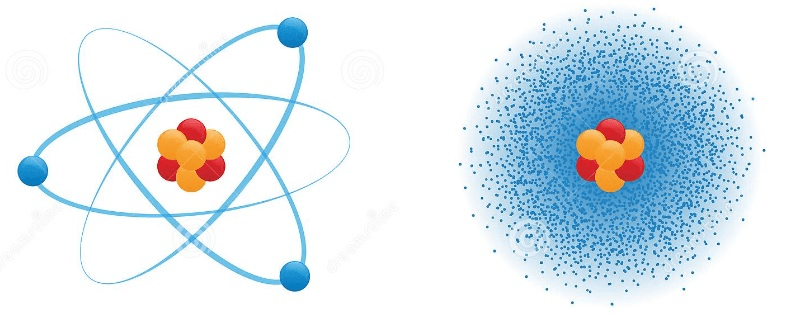
The Electron Cloud
What is the word that refers to different atoms of the same element that have different numbers of neutrons? (p.109)
Isotopes
Which category of elements are essential to the chemicals of life? (p.111)
Nonmetals
What do we call a mixture that has larger parts that are different from each other? (p.117)
In Heterogeneous mixtures, you can see the different parts of the mixture.