Colour Change
Formation of a Gas (bubbling)
Formation of a Solid (precipitate)
Energy Change (light or heat)
Why do elements bond with one another?
For increased stability
HCl, H2SO4 and vinegar are all examples of...
Acids
These pictures are all examples of...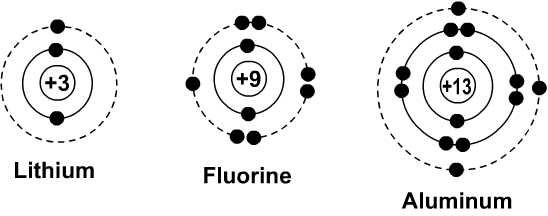
Bohr Rutherford Diagrams
What is this tool?

Scoopula
Law of Conservation of Mass
When a metal loses its electrons to a non-metal it is known as...
Ionic Bonding
In your household, what type of chemicals are primarily bases?
Cleaning products
How many electrons are allowed to be on the 2nd and 3rd rings of a Bohr-Rutherford Diagram?
8 on each
Tip of the inner blue cone.

X H2 + O2 --> 2 H2O
What number must be replace X?
2
When 2 non metals share their electrons it is known as...
Covalent bonding
What do acids taste like?
Sour
The following are examples of...

Lewis diagrams
What tool should be used to measure a specific amount of water?
Graduated cylinder
What are the numbers necessary to balance with equation?
W MgCl2 + X LiF --> Y MgF2 + Z LiCl
W = 1
X = 2
Y = 1
Z = 2
These special types of electrons are the ones lost or shared during bonding...
If experiencing an acid burn, what standard household chemical should you use to stop the burn?
Baking soda
The dots on a Lewis Diagram represent what.
Valence Electrons
The proper way to test the odor of a chemical is known as...
Wafting
What are the 5 basic types of reactions? 200 points for each correctly named.
Synthesis
Decomposition
Single Displacement
Double Displacement
Combustion
Sulphate (SO4-2) Phosphate (PO4-3) Nitrate (NO3-1) and Hydroxide (OH-) are all known as polyatomic ___________...
Ions
When an acid mixes with a base, what type of reaction occurs and what is always created?
Neutralization always creates water
What is the simplest way to figure out the amount of valence electrons on an atom without drawing a B-R diagram.
What is the full name of this tool?

Erlenmeyer Flask