This molecule is known as the universal solvent.
What is water.
Name of the following:
SnO2
What is tin (IV) oxide?
AB -> A + B
What is a decomposition reaction?
Chemical formula for sulfuric acid.
What is H2SO4?
The molar mass of water.
What is 18.02 g/mol?
Name this:
NH3
What is nitrogen trioxide
The most common valence charge for copper.
What is +1
The type of reaction that typically forms a precipitate.
What is double displacement?
The conjugate base in this reaction:
HSO4- + H2O --> SO4- + H3O+
What is SO4-?
Name of this compound:
CuSO4 . 5H2O
What is copper (II) sulfate pentahydrate?
The chemical formula for diphosphorus heptaiodide
What is P2I7?
The chemical formula for aluminum acetate?
What is Al(C2H3O2)3?
The type of reaction that occurs as a result of a titration. Be specific!
What is neutralization.
This is the conjugate acid of water.
What is hydronium ion (or H3O+)?
If pressure is increased, this would happen to the volume of a gas.
Name of this law.
What is decrease? What is Boyle's Law.
The atom that has greater electronegativity in the following molecule:
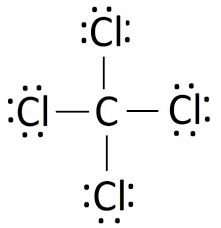
What is chlorine?
The number of electrons gained by phosphorus to bond with magnesium.
What is 3?
The type of reaction that occurs when a fuel combines with an insufficient amount of oxygen.
What is incomplete combustion?
The chemical formula for aluminum hydroxide.
What is Al(OH)3(aq)
The atom that has greater electronegativity in the following molecule:

What is a chlorine?
The maximum number of bonds a nitrogen atom can make.
What is 3?
Atoms gain or lose electrons to form these.
What are ions.
Balance:
__ CH4(g) + __ Cl2(g) --> __ CCl4(g) + __ HCl(aq)
__ CH4(g) + 4 Cl2(g) --> __ CCl4(g) + 4 HCl(aq)
_________________ turns fuschia (dark pink) in a __________.
What is phenolphthalein and base.
The number of grams of FeO produced when 1.25g of Fe is reacted with excess O2.
2Fe + O2 --> 2FeO
n=1.25 g/55.85 g/mol = 0.0224 mol
mole ratio = 2/2
m = nMM
= 0.0224 mol x (55.85 + 16.00 g/mol)
= 1.61 g