What are the boiling and freezing point of water?
A) 100 °C, 0 K
B) 0°C, 100 °C
C) 100 K, 0 °C
D) 373 K, 273 K
Choose the technology that you would need so that you could heat a large room in your house, and maintain a constant comfortable temperature in that room.
A) a gas furnace
B) a wood-burning fireplace
C) an electric fireplace
D) a digital thermostat
One litre of pure water at 30°C is mixed with one litre of pure water at 50°C. The temperature of the mixture will be
A) more than 50°C but less than 80°C
B) 80°C
C) between 30°C and 50°C
D) 20°C
Which of the following is true during the phase change?
A) The average speed of the particles are not changing
B) The total energy of the substance is changing
C) It is the arrangement of the particles that are changing
D) All of the above
Trees transpire large amounts of water through pores in their leaves called stomata. The faster-moving particles escape into the air. As the high-energy particles leave the surface of a liquid, the remaining liquid is cooler than the original liquid. The cool liquid then cools the surface on which it is resting. Scientists call this phenomenon ___________
A) condensation
B) evaporative cooling
C) photosynthesis
D) convection cooling
Which of the following states of matter have particles that do not have a definite shape and do not have a definite volume?
A) solid
B) liquid
C) gas
D) gas and solid
Jake says his thermometer reads 65 degrees celsius. What is the the temperature in the kelvin scale?
A) 200 K
B) 100 K
C) 338 K
D) -208 K
When a popsicle melts completely into a liquid state, the particles _____.
A) gain thermal energy and speed up
B) in the popsicle gets destroyed
C) lose thermal energy and slow down
D) have the same amount of energy
During a phase change, the temperature remains the same, so the particles have ...
A) less average energy
B) more average energy
C) the same average speed
D) a faster speed
Steven notices that the pipelines are missing expansion joints. As a result, which of the following would occur to the pipelines on a very hot summer?
A) expansion
B) contraction
C) rusting
D) melting
Which of the following is NOT part of the particle model of matter?
A) The particles are always in motion
B) The particles have spaces between them
C) All substances are made up of tiny particles
D) As the particles spread away from one another they split
On top of Mount Everest, the boiling point of water is ______ 100 °C because as altitude increases, air pressure __________.
A) below, increases
B) below, decreases
C) above, increases
D) above, decreases
The thermal energy of a substance is the ...
A) total kinetic energy of all the particles
B) average kinetic energy of the particles
C) kinetic energy of each particle separately
D) measure of how hot or cold the substance is
When a substance undergoes a change of state, energy is involved. Which change of state involves a release of energy?
A) melting
B) sublimation
C) evaporation
D) solidification
What is a bimetallic strip?
A. A wire that sends electrical currents to a responder.
B. two strips of metals, curved into a spiral. One metal expands more than the other, leading to operation of an electrical switch.
C. A rock, fashioned into a strip that can tell the temperature.
D. two wires made of different metals that lead to generation of current when heated, thereby operating a switch on or off when the temperature changes.
Liquids ——-definite shape and ———- definite volume.
A) have, do not have
B) do not have, do not have
C) do not have, have
D) have, have
The temperature of a substance is the ...
A) total kinetic energy of all the particles
B) average kinetic energy of the particles
C) kinetic energy of each particle separately
D) measure of how hot or cold the substance is
Which of these has the most thermal energy?
A) house (70° C)
B) match (500° C)
C) house (50° C)
D) match (100° C)
Water appears on the outside of your glass on a hot day, what change of state is this:
A) condensation
B) solidification
C) vaporization
D) fusion

At which temperature do both the solid and the liquid phase of water exist?
A) 273 K
B) 0 K
C) 100 K
D) 373 K
Which of the following would be an example energy transfer?
A) thermal energy is converted into electrical energy
B) thermal energy is transferred from a heater to a room, so it can be heated
C) an ice cube loses thermal energy when it melts in hot lemonade
D) thermal energy is lost when iron melts
The official unit of heat energy is the ...
A) watt
B) joule
C) kilowatt
D) Celsius
Identify the change in state that occurs when CO2(s) changes to CO2 (g) as it is heated.
A) condensation
B) freezing
C) vaporization
D) sublimation
A chemist heated was heating a solid. He realized that by point C, the substance is completely ____________ and is now in the ___________ state.
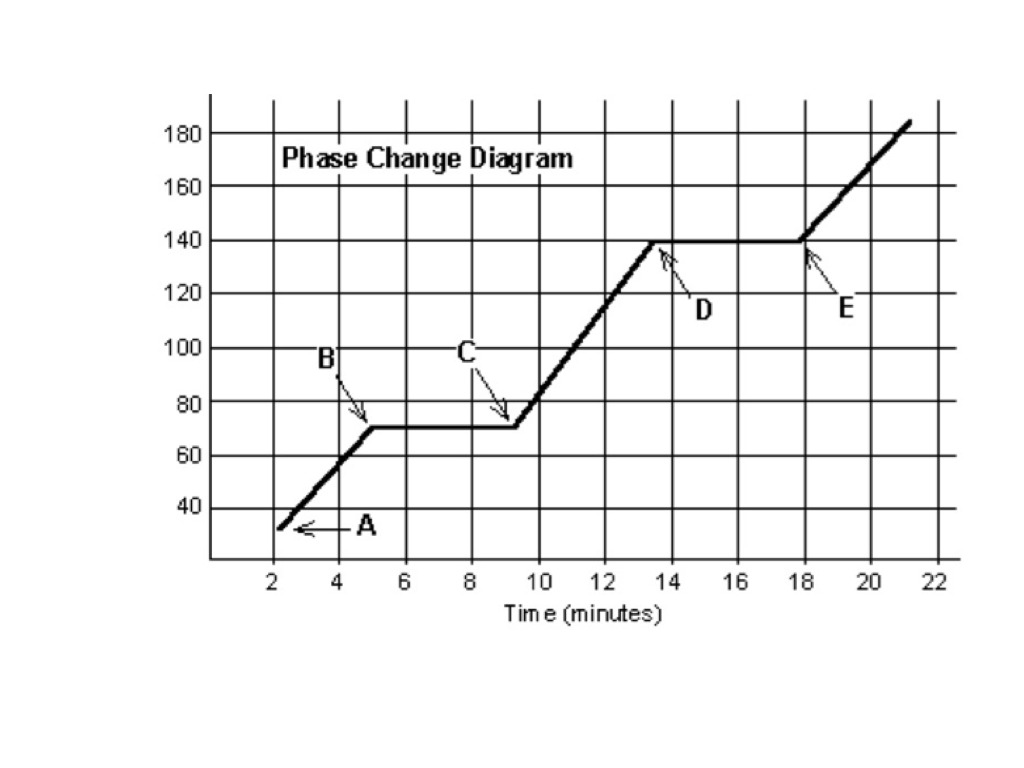
A) Non-newtonian fluid
B) Plasma
C) Bose-einstein condensate
D) Newtonian fluid
According to the particle model of matter, what happens to the motion of particles when the temperature increases?
A) the motion of the particles decreases
B) the motion of the particles increases
C) the motion of the particles is unaffected
D) the energy of the particles decreases as their motion increases
Certain materials are found naturally in the environment. Chlorine is found most often in the gas state. To change it to a liquid, which of the following has to occur?
A) add heat
B) remove heat
C) increase its temperature
D) maintain its temperature
As a substance changes from a liquid to a gas, the average distance between molecules…
a) decreases
b) increases
c) remains the same
d) increases then decreases
The device used to measure the temperature of the object by photographing it using special films or electronic sensors that display images with various colours and brightness on television screens is known as
A) Recording thermometer
B) Thermocouple
C) Infrared Thermogram
D) Analogue thermometers