Aluminum is pounded into thin sheets to make aluminum foil you use in cooking. This represents this physical property.
What is malleability?
This is the Lewis Dot diagram for Carbon.
C with 4 valence electrons
The forces between particles are relatively weak for this state of matter. (lots of space between particles!)
What is gas?
What element is represented by the atom in the picture?
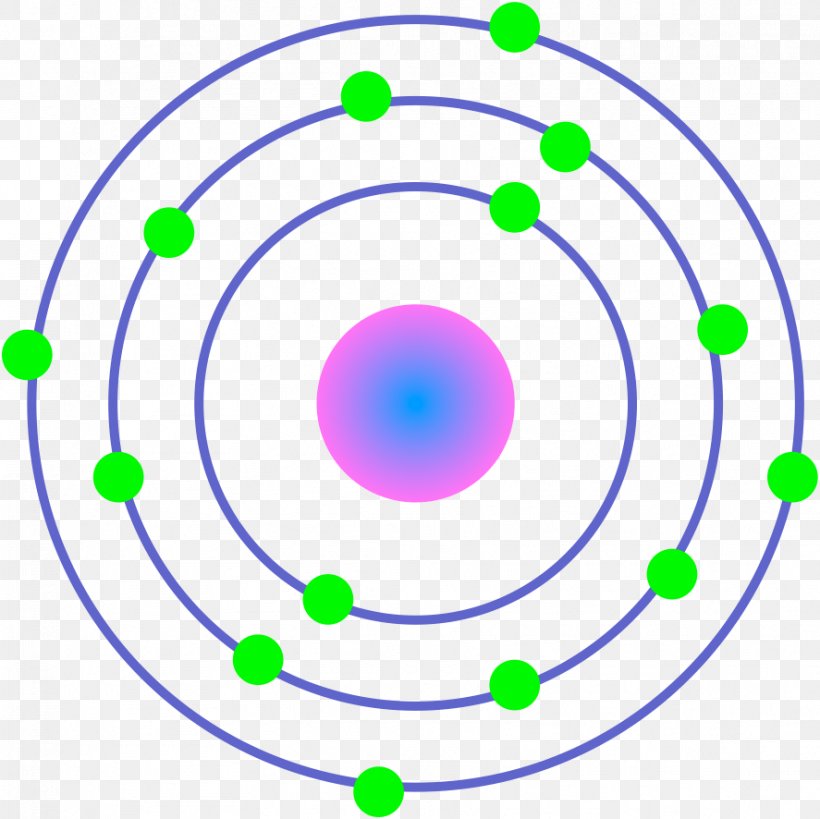
Silicon
State the ion name, formula and whether it will become an anion or cation for Magnesium
magnesium ion, Mg2+, cation
(NH4)2O is made up of this many atoms in total.
State the number of atoms per element as well.
What is 11? N = 2, H = 8, O = 1
Determine the number of atoms in 2 BaBr2
Ba = 2, Br = 4, 6 atoms!
Copper, tin, and gold are
Metals
________________ are good conductors of heat and electricity
metals
What is the ionic compound for Aluminum and Sulfur
Al2S3
A physical change would include:
Change in appearance: Cutting, Crushing, Change in state of matter, Melting, Boiling, Freezing, No new substance formed
Group 7 goes by this name.
What is a halogen?