Tightly packed and vibrates in place
Solid
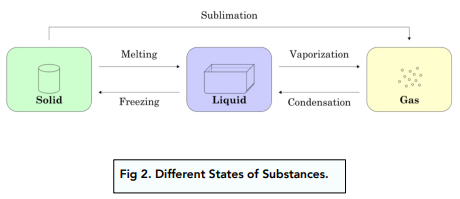
What are the names of the change of state processes?

(A) melting (B) Boiling/Evaporation (C)freezing (D) condensation
What element is represented by the symbol Na?
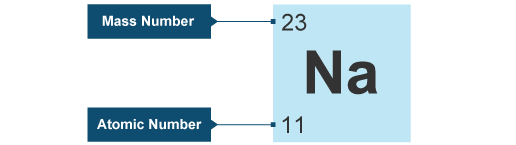
Sodium
On the periodic table elements are arranged in increasing order of ______________ number.
Atomic Number
Recite the first 10 elements on the Periodic Table in order.
Osmosis and diffusion provide evidence for this theory.
PARTICULATE THEORY OF MATTER


Which of the is NOT an example of sublimation?
(A) dry ice to carbon dioxide (B) napthalene to air
(C) iodine crystals to purple vapour (D) water to water vapour
(D) water to water vapour is an example of boiling/evaporation

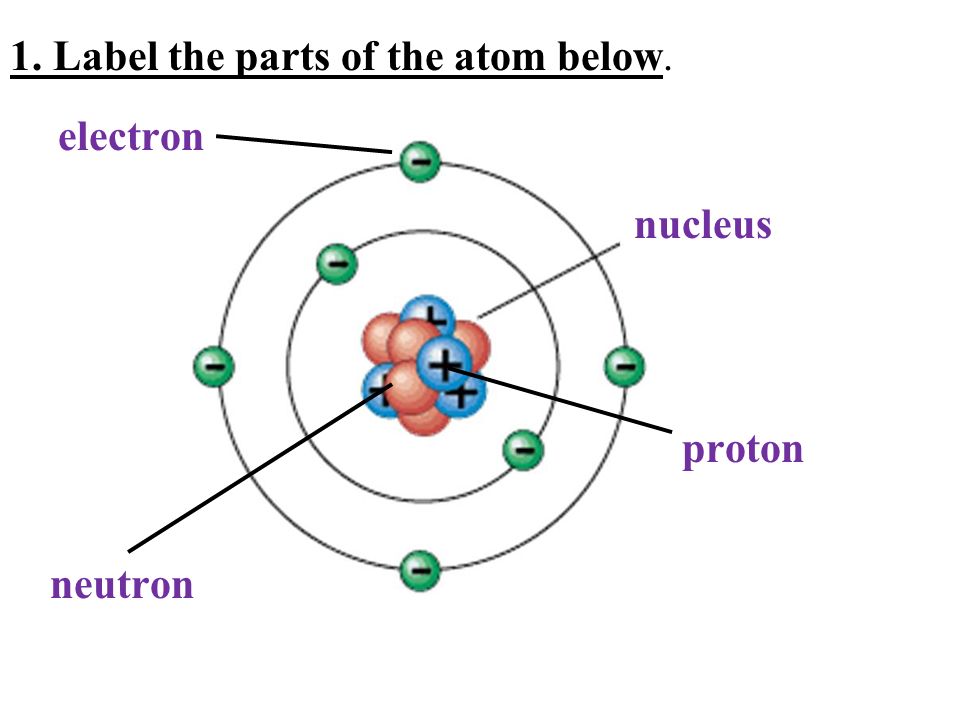
LABEL THE PARTS OF THE ATOM

(A)shell (B) nucleus (C) electron (D) proton (E) neutron
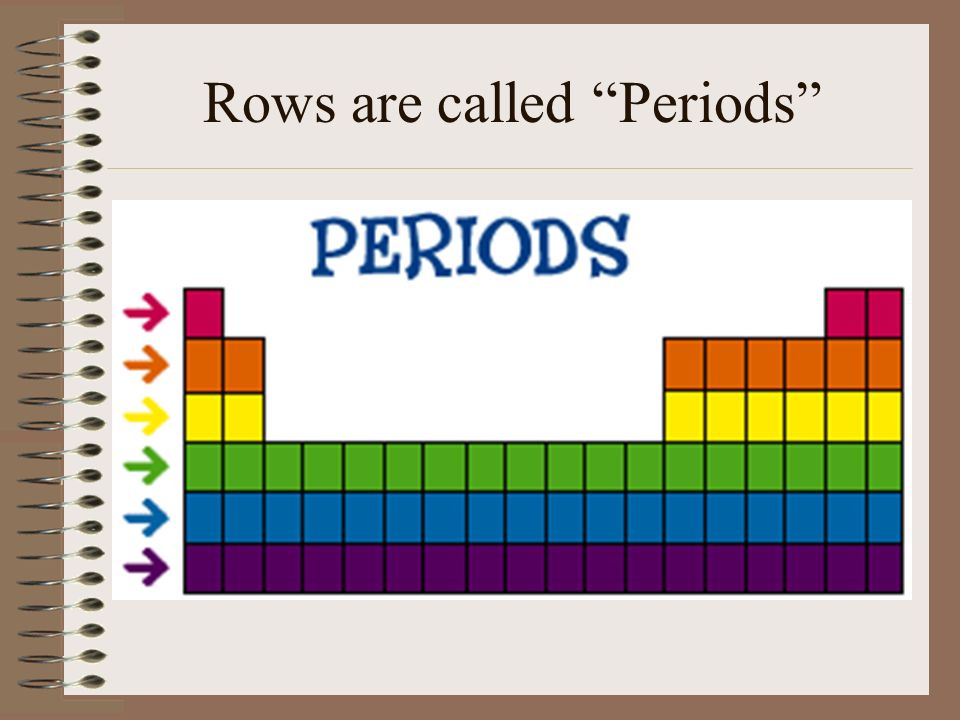
Vertical columns of the periodic table are called _________________ while horizontal rows are called __________________.
Vertical columns: GROUPS
Horizontal rows: PERIODS

Diffusion occurs when there is a difference in concentration from one area to another. Which diagram shows the highest concentration of particles?

There are more particles in diagram A.
Rank the following group of substances in the correct order from the strongest forces of attraction to the least.
(A) water, oxygen, chalk
(B) salt, juice, wind
(C) nitrogen, milk, sugar
(D) air, salt, oil

STRONGEST--> INTERMEDIATE --> WEAKEST
chalk --> water --> oxygen
salt --> juice --> wind
sugar --> water --> nitrogen
salt --> oil --> air
A dish containing butter was left on a sunny windowsill for a few hours. State TWO ways in which the particles of the butter change?
Particles would gain energy, move faster, move further apart and form the liquid state
Calculate the number of protons, neutrons and electrons in the following atom.
Mass number = 136, Atomic number = 56
Therefore,
# of protons = atomic number =56
# of electrons = # of protons = 56
# of neutrons = mass # - atomic # = 136 -56 = 80 neutrons
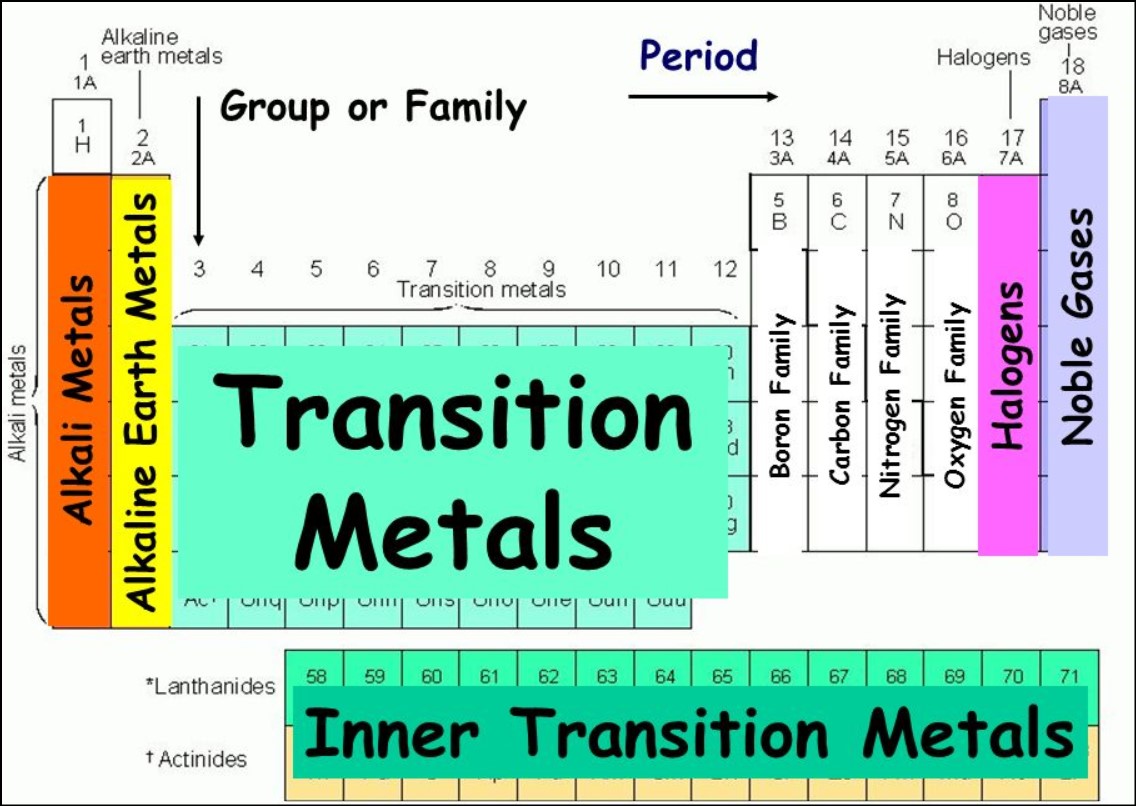
State the family name given to the following Groups on the periodic Table
Group 1
Group 2
Group 7
Group 0
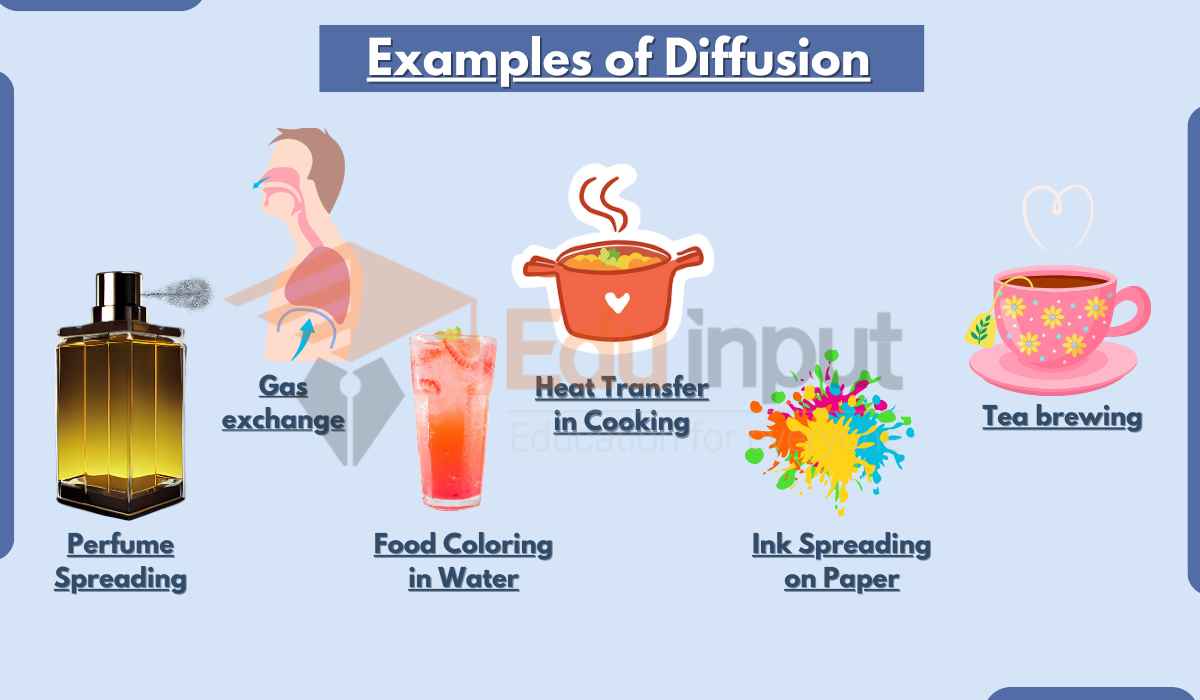
State an example of diffusion that occurs on everyday life
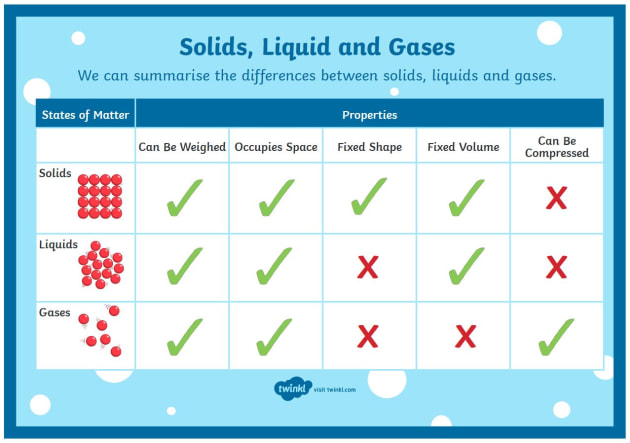
State the property in which liquids and gases have in common?
Both gases and liquids do not have a fixed shape, additionally since they are both states of matter they also have mass and take up space.
What is the boiling point of the following substance?
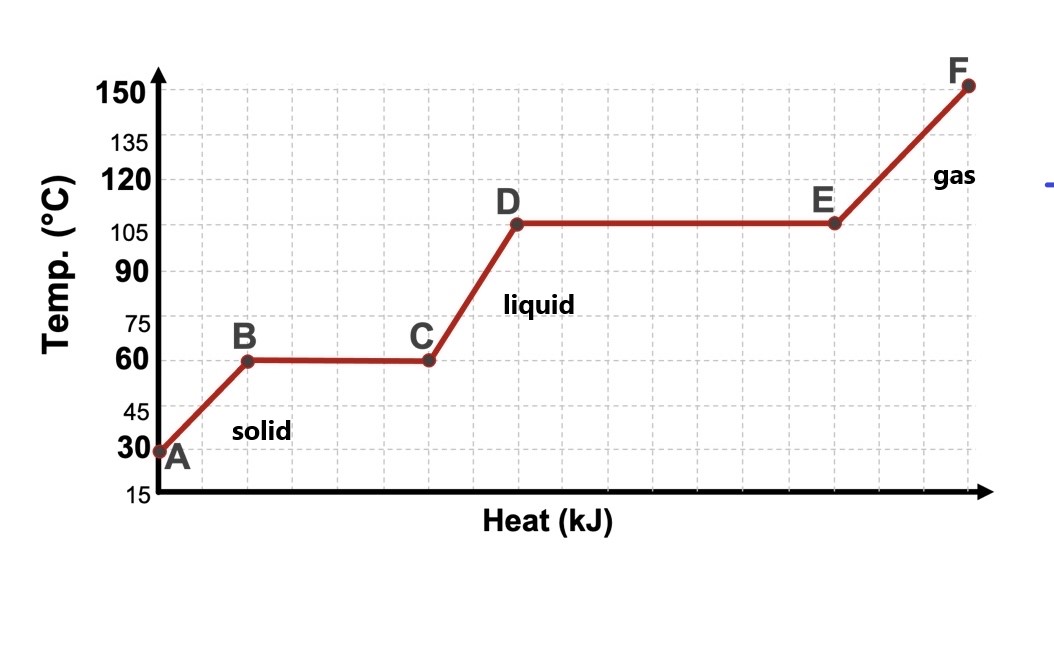
105oC
Draw the atom for the element with the following symbol.
Based on the symbol, potassium will contain 19 protons, 19 electrons and 20 neutrons

What is the electronic configuration of an element X which is found in Group IV and Period 3?
2, 8, 4
Atoms have no electric charge because.....
Atoms have an equal number of negatively charged electrons and positively charge protons. The opposite charges cancel each other.
Three equally sized potatoes were placed in different solutions and left for the same time. One was placed in distilled and the other placed in concentrated solution. What causes the potato strip in beaker 2 to decrease in size ?
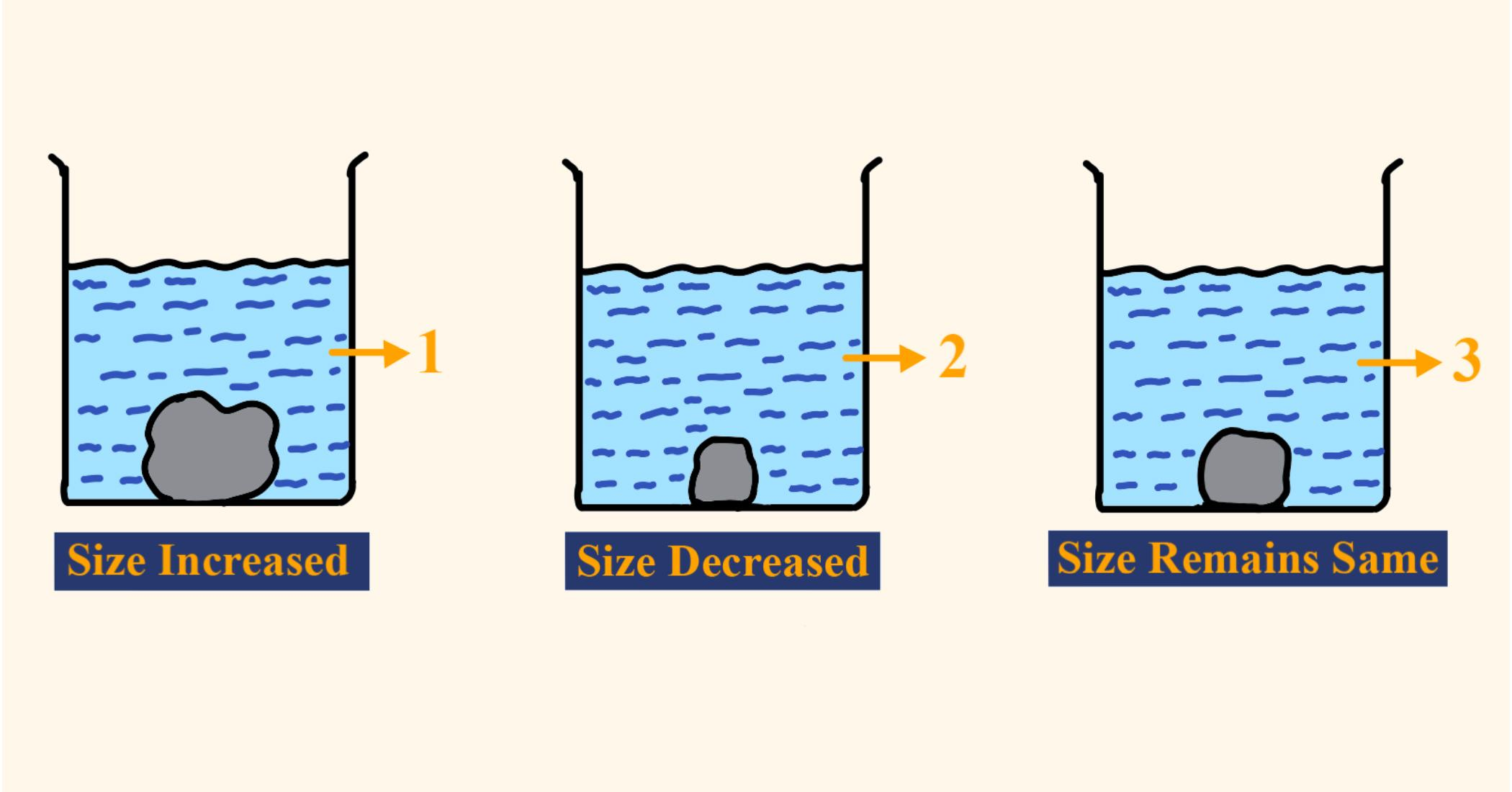
Water moves out of the cell of the potato in beaker 2.
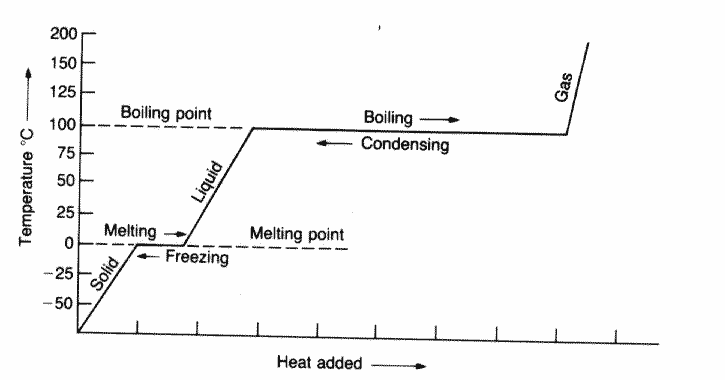
Evaporation and boiling both involve a change of state from liquid to gas. State 2 differences between evaporation and boiling

In what ways are the atomic structures of the following carbon isotopes similar and different?

SIMILARITIES : (1) Same number of protons (2)Same number of electrons (3) All atoms have an overall charge of zero
DIFFERENCES: (1) Different number of neutrons (2)Different mass number (3) The nucleus of carbon-12 is more stable than carbon-13 and carbon-14
Given the electronic configuration of an element X as 2,8,8. State the group and period number to which it belongs.
Element X belongs to group 8 or group 0.
Element X belongs to Period 3.
State two elements that belong to the same group and two elements that belong to the same period
(142).jpg)
