What is the difference between a law and a theory?
A law describes what happens through summary
A theory explains why something happens
How are elements arranged on the periodic table?
(Two Ways)
In order of increasing atomic number (number of protons) and increasing atomic mass.
What causes something to be reactive?
High melting and boiling points, Solids at room temperature, made with a metal and a non-metal, Good conductors of electricity, Distinct crystal shape
What molecule (protons, electrons, or neutrons) determines the element?
Protons
What is a group of flamigos called?
Flamboyance
What is electrolysis? What would it do to water?
When we pass an electrical current
through a chemical compound to cause
it to break apart or decompose
What (2 items) does the atomic number of an element tell us?
It tells us the number of protons in one atom of the element. It also tells you the number of electrons before the atom has bonded to anything.
How many valence electrons does iodine have?
Seven
Why do we use the term average atomic mass when describing elements on the periodic table
Depending on the number of neutrons the mass may vary
You are on your way to visit your Grandma, who lives at the end of the valley. It's her birthday, and you want to give her the cakes you've made.
Between your house and her house, you have to cross 7 bridges, and as it goes in the land of make believe, there is a troll under every bridge! Each troll, quite rightly, insists that you pay a troll toll. Before you can cross their bridge, you have to give them half of the cakes you are carrying, but as they are kind trolls, they each give you back a single cake.
How many cakes do you have to leave home with to make sure that you arrive at Grandma's with exactly 2 cakes?
2
Who was a big contributor to the development of
the law of definite composition
conservation of mass
(Double points if you describe what they are)
Antoine Lavoisier
Conservation of mass: The total mass of the new substance is the same as the original in a true closed system.
Law of definite composition: Compounds are pure substances made of two or more elements in fixed proportions
An element has a atomic mass of 16, and contains 8 protons. What would the bohr model look like?

An element has 16 protons and an atomic mass of 33, how many neutrons does it contain?
17 neutrons
A sundial is a timepiece that has the fewest number of moving parts. Which timepiece has the most moving parts?
Hourglass
List the order of the main five atomic models that we have learned about
Dalton, Thompson, Rutherford, Bohr, Schrodinger
What is the name given to the most reactive group of non-metals and where is the group found in the periodic table?
Bonus points if you explain why they are
The Halogens - group 17
What is the difference between the reactivity in a metal element and the reactivity in a non metal
Non metal will attract an electron while a metal while lose an electron
What is the weight of a proton?
Why should you get these points
Explanations vary
Who do you believe had the biggest impact on the development of the atomic model?
Convince me
Name 4 physical properties of all metals, and show where they are on the periodic table.
Metals are shiny, malleable, ductile, and conductors of electricity.
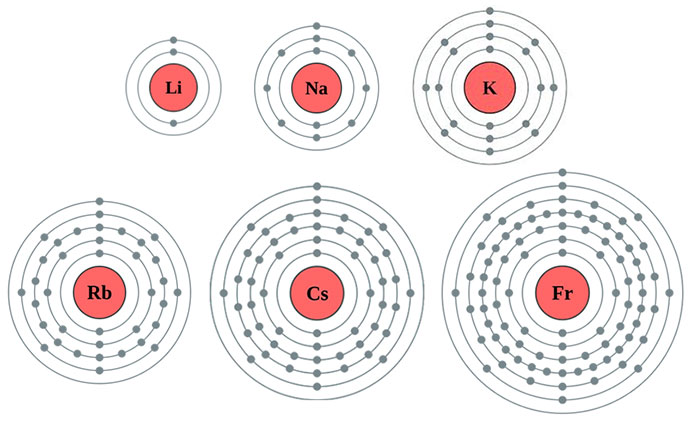
Lithium, explanation works
Draw and label all five atomic models we have learned about on the board
Check answer
Why do we die of old age?
Telomeres