Explain the theory of continental drift.
What is when all the continents were connected (Pangea)?
This changes the shape and size of a rock without changing its composition.
What is weathering?
The cell cycle stage in which the cytoplasm splits into two complete daughter cells.
What is cytokinesis?
In this type of cell transport materials get moved across the cell membrane due to a concentration gradient (moves from high to low).
What is passive transport?
The nitrogenous bases of DNA are…..
What are A, T, C, G
Adenine, thymine, cytosine, and guanine
True or False
All types of energy (renewable and non-renewable) is used in different ways to do the same thing, to turn a turbine.
What is true?
This situation would cause a liquid’s viscosity to increase.
what is decrease the temperature?
The theoretical yield of FeCl2 is 20 grams. What is the percent yield of FeCl2 if my actual yield is 11 grams?
What is:
55% FeCl2
The formula for the ideal Gas Law.
What is:
PV=nRT
The three types of tectonic plate boundaries are....
What are Convergent, Divergent, Transform?
The process by which rocks, sediments, or bits of soil are carried away.
What is erosion?
The step in Interphase in which DNA is duplicated/ copied.
What is Synthesis or S-phase.
In this type of cell transport the cell must spend energy in the form of ATP in order to let materials in and out of the cell.
What is Active transport?
The nitrogenous bases of RNA are….
What are G, U, A, C
Guanine, uracil, adenine, and cytosine.
List two types of non-renewable energy.
What is:
Coal
Natural gas
Petroleum
The reason helium is considered a noble gas.
What is because it has a full outer shell of electrons (full valency)?
How many moles of Oxygen (O2) gas are needed to react with 25 moles of ammonia (NH3)?
Molar masses: NH3 = 17.04g, O2=32.0g, NO=30.01g H2O=18.02g
4NH3 +5O2 -> 4NO + 6H2O
What is:
31.25 mol O2
A tank contains three gases: oxygen, helium, and nitrogen. If the partial pressures of the three gases in the tank are 25 atm of O2, 10 atm of N2, 35 atm of He, what is the total pressure inside of the tank?
What is:
70 atm
The type of plate boundary that moves away from each other usually creating rifts or rift valleys.
What is a divergent boundary?
A type of map that shows specific features of landforms including steepness or flatness of the land’s surface using contour lines.
What is a topographic map?
The longest phase of the cell cycle.
What is interphase?
This type of passive transport only pertains to the movement of water.
What is Osmosis?
List the two processes of protein synthesis.
What are transcription and translation?
List two types of renewable sources of energy.
What is
Solar
Geothermal
Wind
Hydropower
Biomass
An example of a chemical property is-
acidity
density
mass
solubility
What is (4) acidity?
How many moles of nitrogen monoxide (NO) are formed, if 30 grams of water (H2O) are produced?
Molar masses: NH3=17.04, O2=32.0g, NO=30.01g, H2O=18.02g
4NH3 + 5O2 -> 4NO + 6H2O
What is:
1.11 mol NO
A gas with a volume of 130ml and a pressure of 3.0 atm is compressed to 50.0 ml. What is the new pressure?
What is:
7.8 atm
The type of plate boundary that slide past each other and usually creates faults and earthquakes.
What is a transform boundary?
The accumulation or laying down of matter by a natural process, as in the laying down of sediment in streams or rivers.
What is deposition?
List all the phases of mitosis in order.
Prophase
Metaphase
Anaphase
Telophase
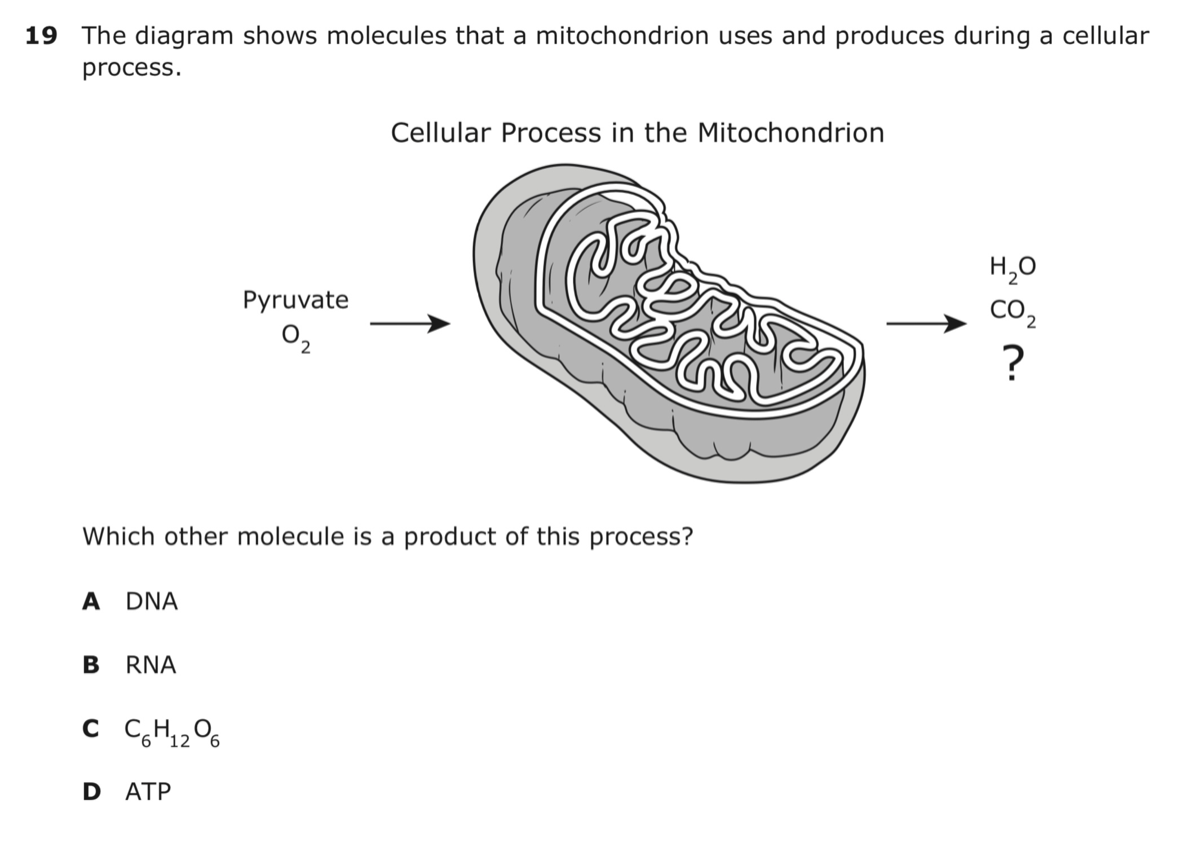
What is answer choice:
D.) ATP
Name the location in the cell where transcription occurs?
What is the nucleus?
These particles are very far away from each other, are easily compressed, and have high energy.
What is a gas?
List the 5 major groups of the periodic table.
What are:
The alkali metals
Alkaline earth metals
Transition metals
Halogens
Noble gasses
How many grams of potassium chloride (KCl) are produced from the decomposition of 35 grams of potassium chlorate (KClO3)?
Molar masses: KClO3=122.55g, KCl=74.55g, O2=32.0g
2KClO3 -> 2KCl + 3O2
What is:
21.29 g KCl
A 0.852-mol sample of a gas is at 288 K and 81.8 kPa. Calculate the volume for the gas.
Remember the gas constant (R) = 8.3145
What is:
24.93 L
The type of plate boundary that moves toward each other and subduction occurs usually causing mountains and or volcanos.
What is a convergent boundary?
When reading a topographic map if contour lines are close together, this means…..
What is the landform is steep?

What is answer choice:
A.) A tumor
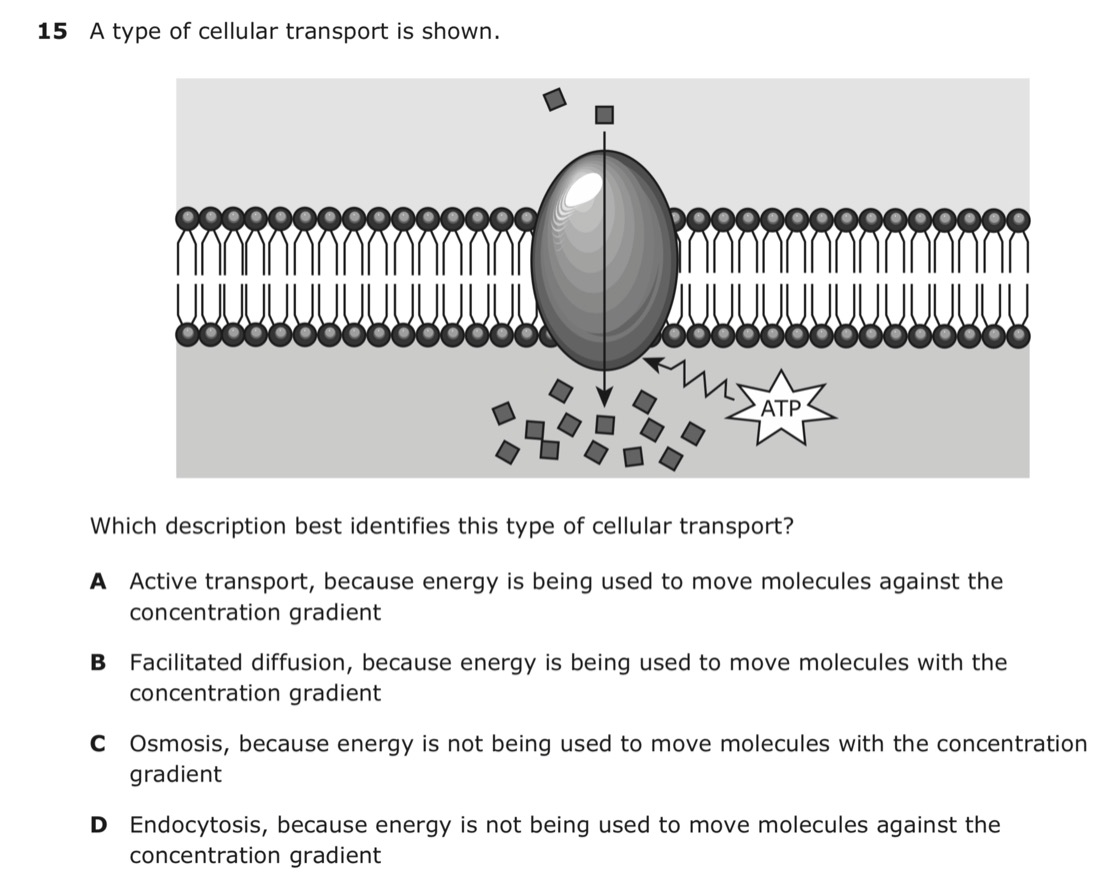
what is answer choice:
A.) Active transport, because energy is being used to move molecules against the concentration gradient.
Transcribe this gene of DNA into mRNA:
TACGCGTTACTCCGTAAAATC
What is:
AUGCGCAAUGAGGCAUUUUAG
Compared to the particles in a gas, the particles in a solid move ....
What is slower than the gas?
A substance has molecules that are loosely bound, allowing them to flow freely around each other. The substance will take the shape of its container, this substance must be a
What is a liquid?
What mass of potassium(K) would react with excess chlorine (Cl) to produce 156 g of potassium chloride (KCl)?
Molar masses: K=39.10, Cl=35.45, KCl=74.55
2K + Cl -> 2KCl
What is:
81.8g K
At constant pressure and 25oC a sample of gas occupies 8.5L. At what temperature will the gas occupy 9.0 liters?
*remember to convert to Kelvin (0oC = 273 K)
What is:
315 K