This type of reaction: 4Fe + 3O₂ → 2Fe₂O₃
What is synthesis?
The molar ratio of the following reaction:
H2O + CO2 --> C6H12O6 + O2
What is 6:6:1:6
The thermodynamic classification of this reaction:
2 Na (s) + Cl2 (g) → 2 NaCl (s) + heat
What is exothermic?
This would increase the reaction rate of the reaction below based on states of matter:
C12H22O11(s) + H2O --> C12H22O11(aq)
what is increase surface area?
When performing gas law calculations, temperature must always be in this unit.
What is Kelvin/K?
>7 on the pH scale
What is base/ basic/ alkaline
The numbers for each blank:
___B4C3 (s) + ___CO2 (g) → ___B2O3 (s) + ___C (s)
What are 1, 3, 2, 6
This common compound has a molar mass of ~44 g/mol
What is carbon dioxide?
The thermodynamic classification of this reaction:
CaCO3(s) → CaO(s)+CO2(g) ΔH=+177.8kJ
What is endothermic?
This would shift the equilibrium of the equation to the right?
A(g) + 2B(g) <--> C(g) + D(g)
what is increase pressure?
An increase in gas temperature will lead to an increase in one or both of these factors.
What are volume and/or pressure?
This factor makes an acid weak/strong.
What is level of ionization in water?
Fill in the missing states:
FeCl3 (aq) + 3 LiOH (aq) --> 3 LiCl (_)+ Fe(OH)3 (_)
what are (aq) and (s)?
The limiting reactant when you mix 35 g of methane (CH4) and 50 g of oxygen (O2)
What is oxygen?
States of matter at section DE
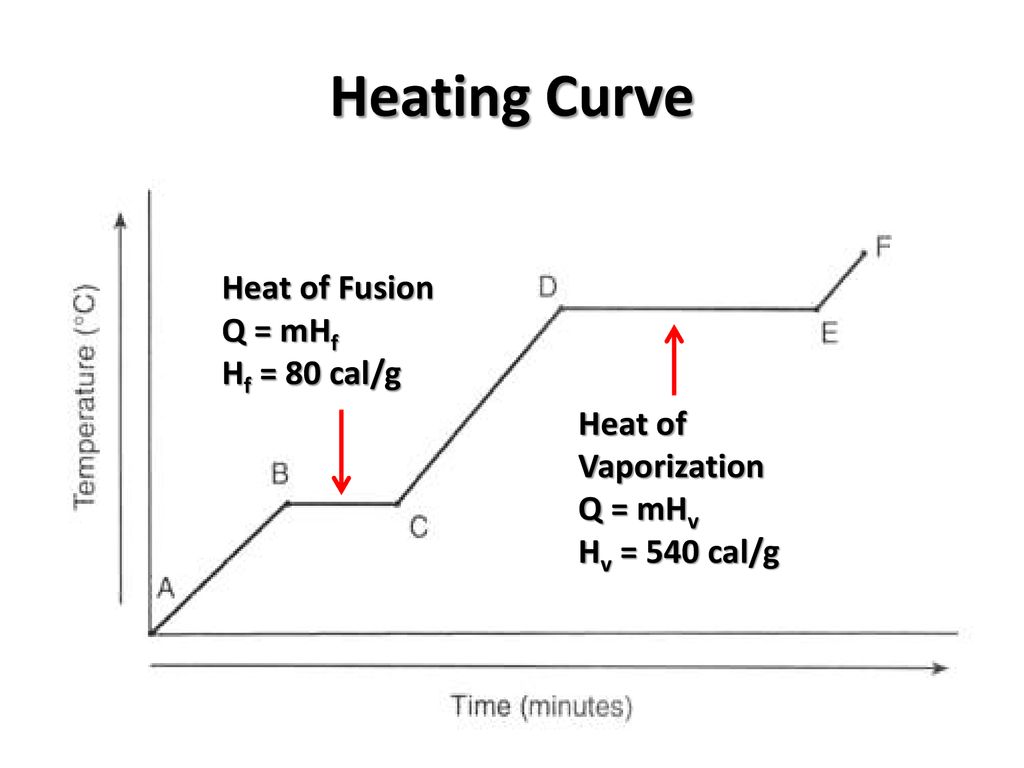
what are gas and liquid?
Adding heat move this chemical reaction in this direction:
Ca(OH)2↽−−⇀CaO+H2O ΔH = 65 kJ
What is forward?
The product of pressure and volume will ___________ if you increase the number of molecules of gas
What is increase?
The concentration of H+ ions in a pH 8.2 solution.
What is 6.3 x 10-9
The likelihood that this will happen:
2 HBr + Cl2 --> 2 HCl +Br2
High. Cl more reactive than Br
The moles of CO2 produced from combusting 570 g octane (C8H18) with excess oxygen
What is 40 moles?
Energy required to melt 50 g of this substance
What is 4000 cal?
The equillibrium constant of the following reaction:
2SO2(g) + O2(g) ⇌ 2SO3(g)
[SO2] =3.0×10-3 M
[O2] = 3.5×10−3 M
[SO3] = 5.0×10−2 M
What is Keq =79,365
A 4 liter oxygen tank contains 19 moles of oxygen gas at 150 atm. The volume of the same amount of gas at standard pressure (1 atm) would be:
What is 600 L?
This will make water very conductive, react strongly with magnesium, and produce a low pH color on a test strip.
What is a strong acid?
The likelihood that this will happen:
Cu + Mg(NO3)2 --> Cu(NO3)2 + Mg
what is not likely! Mg more reactive than Cu
The grams of H2O produced from combusting 0.40 g butane (C4H10) with excess oxygen
What is 0.62 g?
The specific heat of water is 4.28 J/goC How much energy does it take to heat a pot of water (1200 g) from room temp (20oC) to boiling (100oC)?
What is 410,880 J?
These LeChatlier stresses are employed in the Haber Bosch Process, which moves the following reaction forward:
N2(g) + 3H2(g) ⇌ 2NH3(g).
What is high temp, high pressure, catalyst
The number of gas molecules present in a 10 L sample of atmospheric gas at 20oC and 1 atm.
R = 0.0821 L*atm/mol*K
What is 2.5 x 1023 molecules?
The conjugate acid in this equation:
HBr + NH3 <==> NH4+ + Br-
What is NH4+?