According to the Octet Rule, atoms have the tendency to reach stability by achieving how many valence electrons?
Eight (8)
What do we call the separation of electric charge leading to a molecule having a dipole moment?
Polarity
Bonds which are found within molecules are called what?
Intramolecular bonds
What are the forces found between molecules?
Intermolecular forces (IMF)
What is the theory used to predict molecular shape based on electron pair repulsions?
VSEPR Theory
Draw the correct valence electrons on this lewis structure:
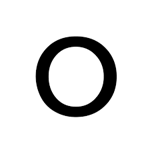
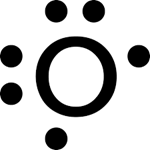
A bond between atoms with a 0.2 electronegativity difference is considered what type of bond?
Nonpolar covalent
H2O
Yes
What is the weakest type of IMFs found in the list below?
Hydrogen Bonding
London Dispersion
Dipole-Dipole
Ionic Forces
London Dispersion
What type of IMFs are found in diamonds? (Hint: the strongest type of IMFs)
Network covalent
Draw the lewis structure for the compound:
H2S

What is the difference in electronegativity of the following bond?
Cl-O
|3.5-3.0| = 0.5
What state of matter does NOT have intermolecular forces?
Gas
What is the strongest type of IMFs found in the list below?
Hydrogen Bonding
London Dispersion
Dipole-Dipole
Ionic Forces
Ionic Forces
Is the following molecule symmetrical or asymmetrical?
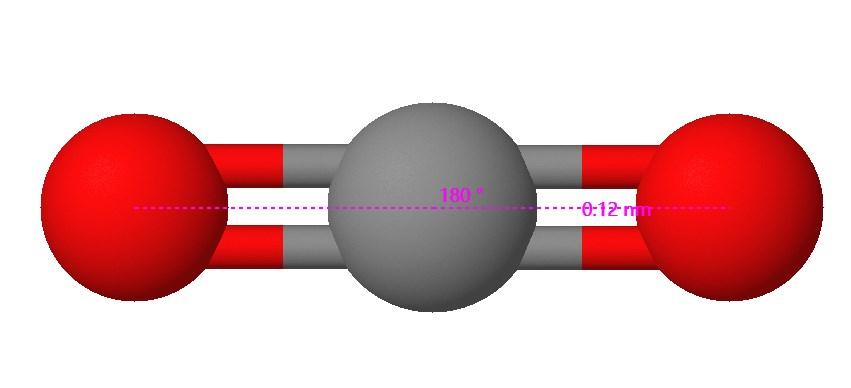
Symmetrical
What is the molecular shape for the compound below?

Trigonal Pyramidal
Is the molecule below polar or nonpolar?

Polar covalent
Does the following have intramolecular bonds, IMFs, both, or neither?
H2O liquid
Both intramolecular bonds and IMFs
What type of IMFs are found in the following compound?
NaCl
Ion-Ion (Ionic)
What type of IMFs are found in the following compound?
CH3OH

Hydrogen Bonds
London Dispersion
What are the bond angles for the molecular geometry of H2O?
<109°
Determine if the following bond is nonpolar, polar, or ionic based on electronegativity difference:
C-O
|3.5-2.5| = 1.0
Polar covalent
Does the following have intramolecular bonds, IMFs, both, or neither?
CH4 gas
Intramolecular bonds
What type of IMFs are found in the following compound?
CH3Br
London Dispersion
Is the following molecule symmetrical or asymmetrical?
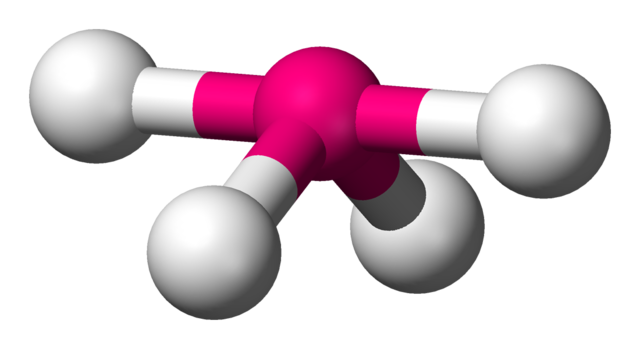
Asymmetrical