The subatomic particle located outside the nucleus of an atom
What is an electron?
The symbol for Hydrogen
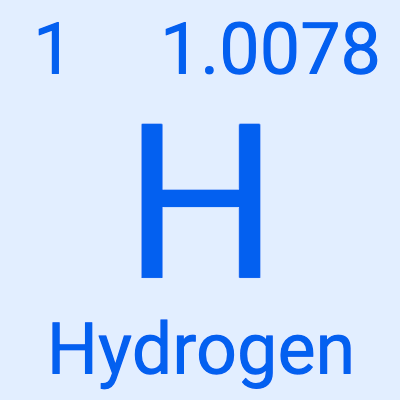
What is letter "H"?
In a chemical formula, this number shows how many molecules are present (COEFFICIENT) OR (SUBSCRIPT)
What is the coefficient?
List the product(s) in the following chemical equation:
Na + Cl → NaCl
What is NaCl (sodium chloride)?
This type of macromolecule is made up of atoms of Carbon, Hydrogen, and Oxygen. They're formed by green plants in reactions of carbon dioxide and water during photosynthesis.
What are carbohydrates?
The atomic number of an atom equals ...
What is the number of protons?
The atomic number of Calcium is...
What is 20?
 In this chemical formula, the number "3" is the ____.
In this chemical formula, the number "3" is the ____.
What is the subscript?
This law states that atoms are not created or destroyed during chemical reactions.
What is the Law of Conservation of Matter?
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are giant biomolecules made of monomers called nucleotides. They are classified as ______.
Nucleic Acids
The atomic mass of an element is the average number of...
What is the number of protons + neutrons?
"Periods" of the Periodic Table contain elements organized (VERTICALLY) or (HORIZONTALLY)
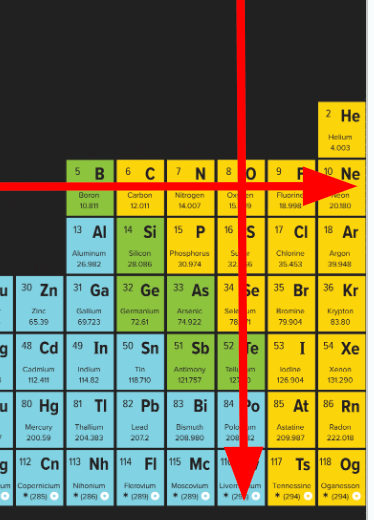
What is "Horizontally"?
What number would replace the "x" in the chemical formula for this molecule HxO?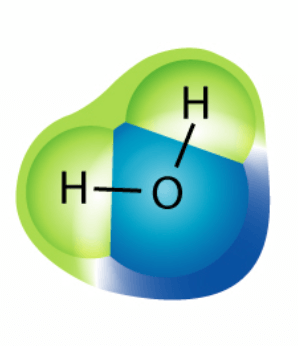
What is the subscript "2"?
H2O
List three pieces of evidence that would indicate a chemical reaction has taken place.
What are:
Color change
Formation of new gas, liquid, or solid
Production of light or heat
The type of macromolecule pictured here forms cellular membranes.
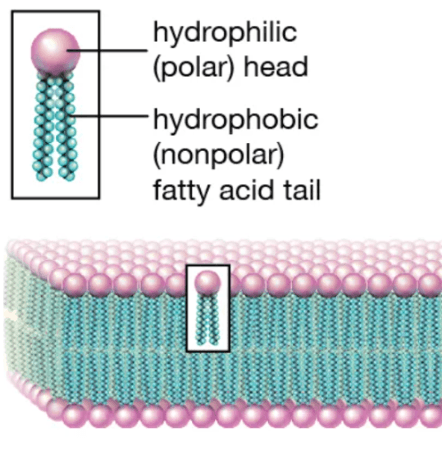
What are "lipids"
These three isotopes of Carbon are different in the number of _____ each atom has.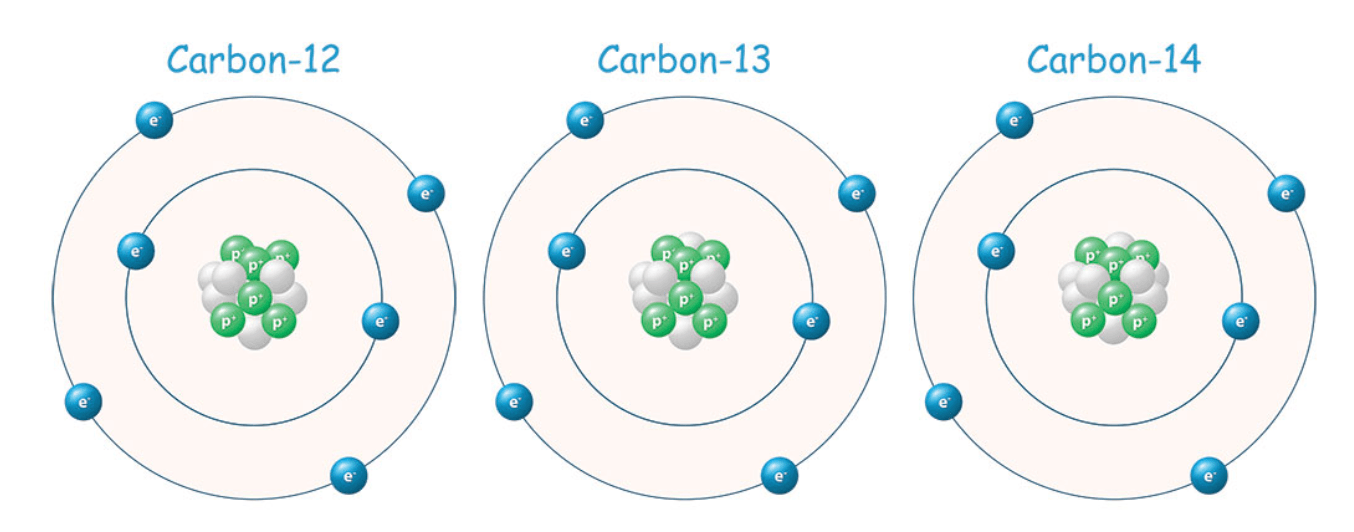
What are neutrons?
Elements on the Periodic Table in (VERTICAL) or (HORIZONTAL) rows have the most similar properties.

What is (Vertical) rows/ Groups?
How many molecules of glucose are represented here: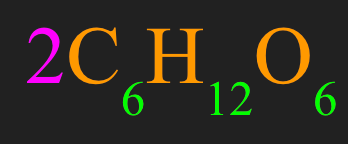
What are two molecules of glucose?
The energy required to start a chemical reaction is called: __________________
What is "Activation Energy"?
This type of macromolecule is made of three components: pentose sugar (5-carbon sugar), phosphate group, and nitrogenous base
What is a "nucleic acid"
The number of neutrons in an atom of the element Iron

What is 30 neutrons?
Which Alkali Metal is pictured reacting with water here? (Li, Na, or K?)

What is K, Potassium?
How many atoms of Hydrogen are contained in the molecules of glucose?
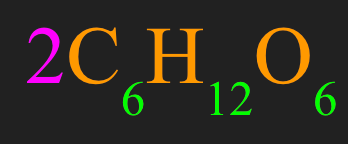
What is 24 atoms of Hydrogen?
In cells, the proteins called, _______, speed up reactions by lowering the activation energy needed to complete a chemical reaction.
What are "enzymes"?
Enzymes are proteins that provide a place for substrates to get together or break apart with less energy required by the body. List three factors that can affect enzyme activity.
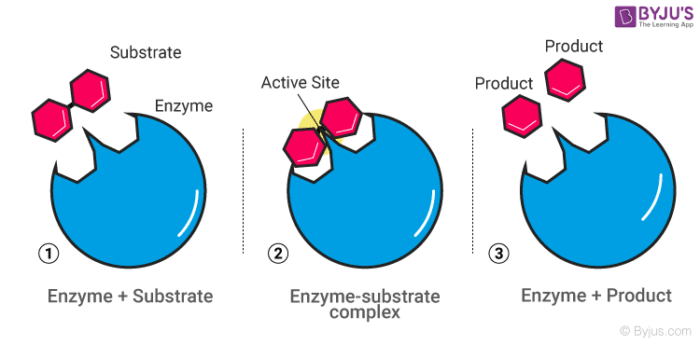
What are:
Temperature
pH
Inhibitors
Amount of substrate
Concentration of enzyme