Did his experiments with oil drops.
Millikan
He used gold foil for his experiments
Rutherford
His experiments involved a cathode ray tube.
Thomson
The particle(s) found in the nucleus is/are the _____
protons and neutrons
The positively-charged particle found in the nucleus is the ____.
proton
Frequency is measured in
Hz=1/s=s-1
Thomson measured the ____at which an electron beam was bent by a magnet.
angle
Millikan determined the ____ of a single electron.
charge
_____dropped electrically charged oil drops between plates at different voltages and adjusted the voltage between the plates until the drops floated.
Millikan
Forms of the same element that differ only by the number of neutrons are called
isotopes
The particle found outside the nucleus in the _____ cloud is the _____.
electron, electron
What measurement is shown in the picture below?
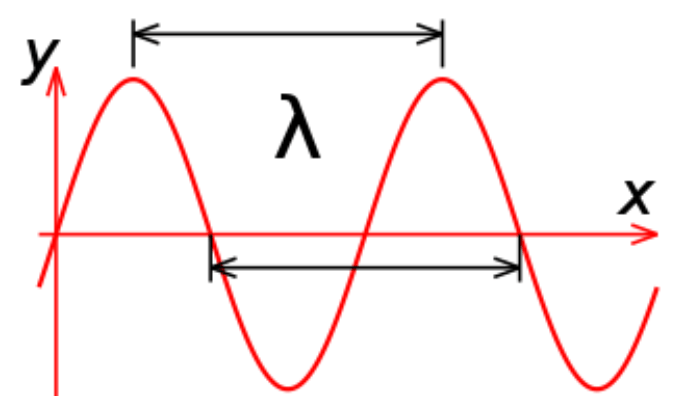
wavelength
_____shot alpha particles at gold foil to discover the atomic nucleus.
Rutherford
The positively-charged particles shot in the gold foil were helium nuclei, also called _____particles.
alpha
Most of the positive particles in the gold foil experiment went straight through the foil, which meant that _____?
An atom is mostly empty space
The number of protons in bismuth-209
83
Protons and neutrons have roughly the same mass of _____u. Electrons have a mass of about _____u.
1 u, 1/1837 u
What is the wavelength of an electromagnetic wave with a frequency of 485 Hz?
618556.701 m = 6.18556701 X 105 m
In the cathode ray tube experiment, the higher the charge, the ____the beam was bent.
more
In the cathode ray tube experiment, the higher the mass, the_____the beam was bent.
less
A few of the positive particles in the gold foil were deflected by a ____, _____, _____-_____ nucleus.
small, dense, positively-charged
The number of electrons in gallium-70
31
Protons and neutrons are held together in the nucleus by _____ _____.
nuclear force
What is the energy of an electromagnetic wave with a frequency of 485 Hz?
3.21361×10−31 J
What was J.J. Thomson able to determine from his experiment?
He was able to calculate the charge-to-mass ratio of an electron.
When plates at different voltages were adjusted to allow electrically charged oil drops between them to float, the force up from _____ was equal to the force down due to _____.
electricity, gravity
What does the animation pictured below show about cathode rays?
It shows that cathode rays (electrons) are negatively-charged because they bend toward the positive side and away from the negative side.
The number of neutrons in cesium-133.
78
Electrons are held into the atom by____.
electrical attraction to the positive nucleus.
What is the wavelength of an electromagnetic wave with an energy of 2.53 X 10-19 J?
7.856916996 X 10-7 m = 785.6916996 nm