The s block has how many lines and arrows when filled?
1 line, 2 arrows
What is the electron configuration of Lithium?
1s2 2s1
What element has the following electron configuration:
1s2 2s2 2p4
What is Oxygen
This is the most important rule in hog Hilton
What is hogs are lazy
What is the value for speed of light
How many protons neutrons and electrons are in Mg-26
12,14,12
The p block has how many lines and arrows when filled?
3 lines, 6 arrows
What is the electron configuration of Carbon?
1s2 2s2 2p2
What element has the following electron configuration:
[Ar] 4s2 3d9
What is Copper
this rule is when electrons must occupy individual orbitals before pairing them up
what is hunds rule
What measurement is shown in the picture below?
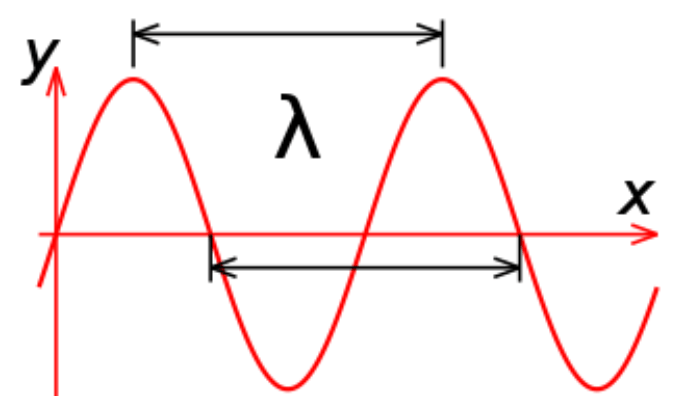
wavelength
How many neutrons are in Ir
115
The d block has how many lines and arrows when filled?
5 lines, 10 arrows
What is the noble gas electron configuration of Arsenic?
[Ar] 4s2 3d10 4p3
What element has the following electron configuration:
[Xe] 6s2 5d1 4f14 5d9 6p2
What is Lead
What is the rule that correlates to hogs not wanting to look each other in the eye
pauli rule
What is the wavelength of an electromagnetic wave with a frequency of 485 Hz?
618556.701 m = 6.18556701 X 105 m
What are the elements called in the last column on the periodic table?
Noble Gases
The f block has how many lines and arrows when filled?
7 lines, 14 arrows
What is the noble gas electron configuration of Titanium?
[Ar] 4s2 3d2
What element ends on the following electron configuration:
4s1
What is Potassium
why is the maximum amount of electrons that can fit in an orbital 2?
What is repulsion
What is the energy of an electromagnetic wave with a frequency of 485 Hz?
3.21361×10−31 J
How man neutrons are in Fe+2
30
what block is astatine located in?
P block
What is the noble gas electron configuration of Osmium (Os)?
[Xe] 6s24f145d6
What element ends on the following electron configuration:
5f7
What is Americium
How were the hogs in Hilton related to our unit?
they represented electrons
What is the wavelength of an electromagnetic wave with an energy of 2.53 X 10-19 J?
7.856916996 X 10-7 m = 785.6916996 nm
How many electrons are in Ca+2
18