Which state of matter has a fixed shape and a fixed volume?
Solid
For the process of melting, does energy need to be added or released from the system?
Added
What is the relationship between Carbon-12 and Carbon-13?
How many neutrons, protons, and electrons are contained in a neutral atom of Chromium-52?
What is the ideal gas law equation?
PV = nRT
Is boiling point an intensive or extensive property?
The atoms of a substance start moving faster. Did the kinetic energy of the atoms increase or decrease? What about the temperature?
Kinetic Energy: increases
Temperature: increases
Is this an example of fission or fusion?

Fission
6329Cu
Copper-63
For ideal gases, do volume and temperature have a direct or inverse relationship?
Direct.
PV = nRT. As V increases, T must also increase.
Ms. Gelfand has a beaker of an unknown green substance.
1. Should Ms. Gelfand use an extensive or intensive property to identify the unknown substance?
2. Name a specific property Ms. Gelfand could use to identify the unknown substance.
1. Intensive
2. Any intensive property (temperature, boiling/melting/freezing point, color, smell, density, taste)
A water bath is at 25oC. An object is placed in the water bath. After 10 minutes, the water bath is at 38oC. Did the object undergo an exothermic or endothermic reaction?
Exothermic
Name 3 uses and/or implications of nuclear chemistry outside of the classroom.
1. Nuclear Medicine (PET scans, radioimaging, radiotracing, cancer treatment)
2. Nuclear energy
3. Smoke detectors
What would happen if one proton was added to an element with 10 protons?
It would change from Neon (Ne) to Sodium (Na).
Think of a real world example that demonstrates the relationship between pressure and temperature.
tire in warm or cold
pot of boiling water
Ms. Gelfand is bad at sharing, and is convinced that the cookies her siblings were given are bigger than the cookie she was given. Knowing that the average cookie is 4 in2, she measures each cookie and gets the following areas for all four cookies: 4.4 in2, 4.1 in2, 3.8 in2, 4.05 in2.
Are these measurements accurate, precise, both, or neither?
Accurate but not precise
What is the difference between heat and temperature?
Temperature is a measure of kinetic energy of a substance
Heat is the transfer of energy between substances
What kind of decay occurs when Gd-159 decays into Tb-159? Write the equation for this decay.
Beta decay
15964Gd → 15965Tb + 0-1e
Write the isotope notation of a carbon and nitrogen atom that share the same number of neutrons.
(Multiple correct answers)
Carbon-12 and Nitrogen-13
Draw two gas particle diagrams: one for a gas at high temperature, and one for a gas at low temperature.
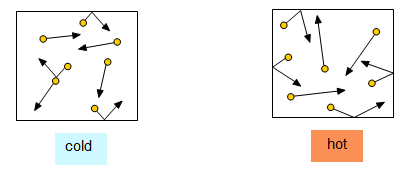
Lower temperature: shorter arrows
Higher temperature: longer arrows
Yesterday, Ms. Gelfand found a rock on her hike that had a mass of 7.45g. She takes a graduated cylinder filled with 10 mL of water, and drops the rock in the water. The graduated cylinder now reads 12.5 mL. What is the density of Ms. Gelfand’s rock? Include units!
2.98 g/mL
You are trying to determine the best material to use to make a hand warmer. You take a calorimeter made out of a styrofoam cup filled with 100 g water at 23oC, and place a 73g sample of CaCl2 inside. You record the temperature inside the styrofoam cup until you notice that the temperature stops changing – at 25.4oC. If the specific heat capacity of water is 4.18 J/goC, what is the change in energy for this reaction?
1,735.5 J
Q = mcΔT = 173g(4.18 J/goC)(25.4oC – 23oC) = 732.3 J
A (fake) element, Washingtonium, has the following percent abundances:
Washingtonium-86: 73%
Washingtonium-87: 22%
Washingtonium-88: 5%
What is the average atomic mass of Washingtonium?
86.32 amu
86 amu(.73) + 87 amu(.22) + 88 amu(.05) = 86.32 amu
What will the charge of an Aluminum atom with 10 electrons be?
+3
What is the pressure of 4.2 moles of oxygen gas at 25oC that occupy a volume of 12.5 L? Give units!
8.2 atm
PV = nRT
P = nRT/V
((4.2 moles)(0.0821 (L*atm)/(K*mol))(298.15 K))/12.5L = 8.2 atm