These are three things you need to wear in addition to long pants, long sleeved shirts, and proper shoes for every lab.
What are gloves, goggles, and aprons?
This is the relationship between pressure and volume.
What is an inverse relationship?
This is the name of the ratio that relates the coefficients of two separate substances in a balanced chemical equation?
What is the mole-to-mole ratio?
This type of reaction always yields carbon dioxide and water.
What is a combustion reaction?
This is how many of anything are in one mole.
What is 6.02 * 1023?
This is what you should do when glassware breaks.
What is stop moving and call over Ms. Nanfara?
This is represented by plateaus on heating or cooling curves.
What is a phase change?
In the reaction below, how many moles of oxygen gas are present?
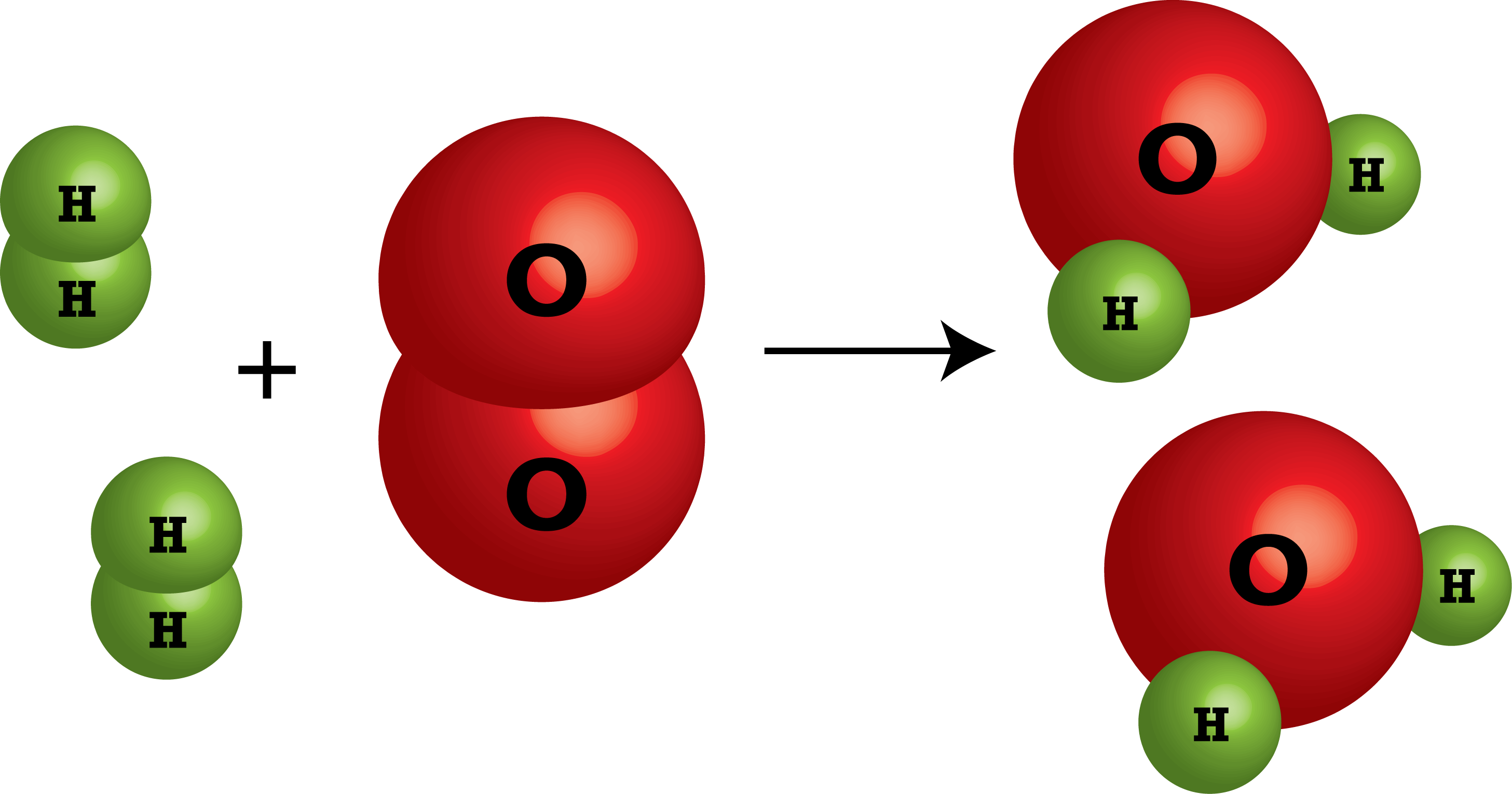
What is 1 mole?
Nitrogen monoxide breaks down into its elements. These are the coefficients for each product.
What is 1 N2 and 1 O2?
This is what we use that relates grams to moles of a chemical formula.
What is molar mass/formula mass?
This is why we never rinse heated glassware right away.
What is cold water will shatter hot glass?
This equation relates moles of a gas to its volume, pressure, and temperature.
What is the ideal gas law? PV=nRT
In the synthesis of oxygen and hydrogen to make water, I start with 5 L of oxygen and excess hydrogen. How many atoms of water will I make?
What is 2.68 * 1023 atoms H2O?
This reaction type requires the use of phase tags to identify if the reaction will happen.
What is a double replacement reaction?
A 4.0 gram sample of grapes contain 61% by mass sugar. How much sugar is in this sample, in grams?
What is 2.5 grams of sugar?
This is what you should do before you weigh anything.
What is calibrate the scale/set it to 0?
This is how we calculate total heat from an object in a single phase as it increases or decreases temperature?
What is Q=mc(Tf-Ti)?
Silver chloride reacts with calcium. If I have 10 grams of each substance, how much calcium chloride will I make?
What is 3.87 grams CaCl2?
Balance the following equation:
P4O10 + H2O --> H3PO4
What is:
P4O10 + 6 H2O --> 4 H3PO4?
A student isolates a molecule and finds the molecule contains 2.8 grams of nitrogen and 6.4 grams of oxygen. This is the molecular formula of the substance.
What is NO2?
This is the purpose of the fume hood.
What is to ventilate chemicals?
A sample of ice is -15oC. It heats up until it boils into a gas at 150oC. How many calculations are needed to find total energy?
What is 5 calculations?
2 grams of mercury(II) acetate are mixed with 3 grams of potassium iodide in a beaker of water. I only collected 85% of the expected amount of mercury(II) iodide. How much mercury(II) iodide did I actually collect?
What is 2.42 grams HgI2?
This law defends the use of coefficients in a chemical equation.
What is the Law of Conservation of Matter/Mass?
Liquid mercury has a density of 13.56 g/mL. If I have 35.0 mL of liquid mercury, how many atoms do I have?
What is 1.42 * 1024 atoms of Hg?