Particles
Particles
Calculations
Readings
Problems
The ________ is the theory that describes all matter as being composed of tiny particles in endless random motion
What is "Kinetic Molecular Theory"?
Pressure is greater at the bottom of the Mariana Trench than the top of Mt Everest because there are _________ pushing down on each other.
What are "Air Particles"?
Between the gas and room pressure, ______ is exerting more pressure in the following manometer
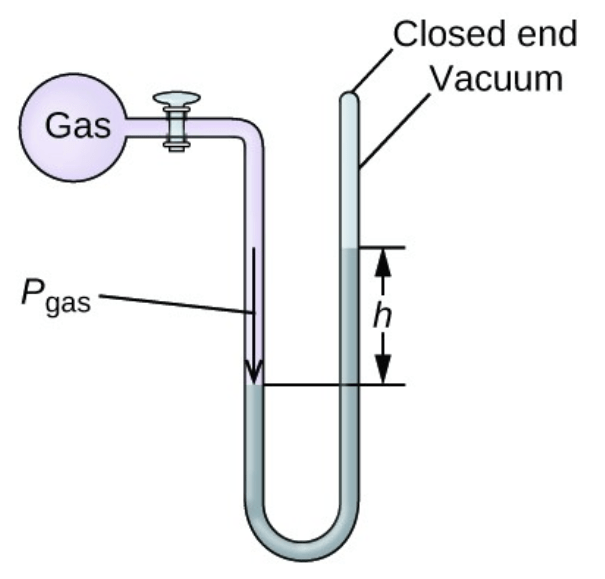
What is "gas"?
The way to calculate total pressure of a tank with various gases in it is to ______ the various gases' pressure values
What is "add"?
What is "Temperature and amount of air?"
What is "increases"?
1.00 atm is also equal to ______ mmHg
What is "760.0 mmHg"?
Between the gas and room pressure, ______ is exerting more pressure in the following manometer
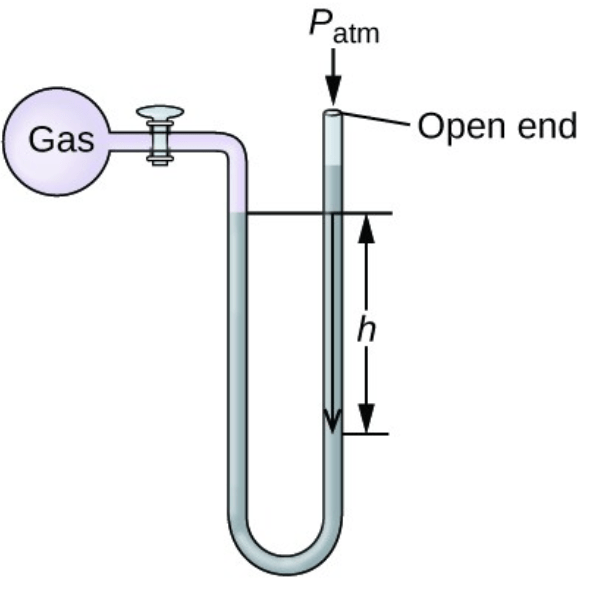
What is "gas"?
The law called __________ states that a container filled with nonreactive gases will equal the added pressure values of each gas
What is "Dalton's Law of Partial Pressure"?
A thermometer reads 37.2 degrees Celsius. The reading in Kelvin is ______ K
What is "310 K"?
If I decrease the size of a container while holding the same number of particles there would be more pressure because more ________ are occurring.
What are "collisions"?
700.0 torr is equal to _____ psi
What is "13.54 psi"?
The height of the gas is 101 mmHg and the height of the vacuum end is 148 mmHg. If the atmospheric pressure is 730 mmHg, then the gas pressure is __________.
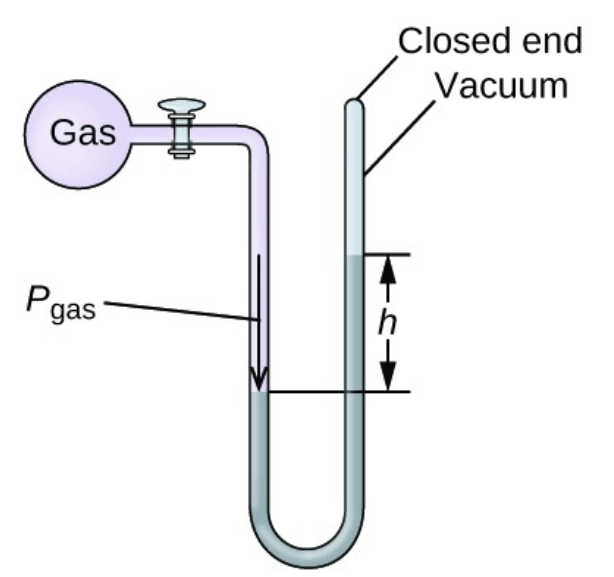
What is "777 mmHg"?
If the total pressure of a tank is 50.0 atm, the pressure of Helium inside is 25.0 atm and the pressure of Nitrogen inside is _____ atm
What is "25.0"?
A tire contained 10.0 L of air at 14.7 psi. After an hour of driving, the tire only contains 8.9 L at _____ psi
What is "16.5 psi"?
The diagram on the left shows a container that is experiencing less _________ than the one on the right
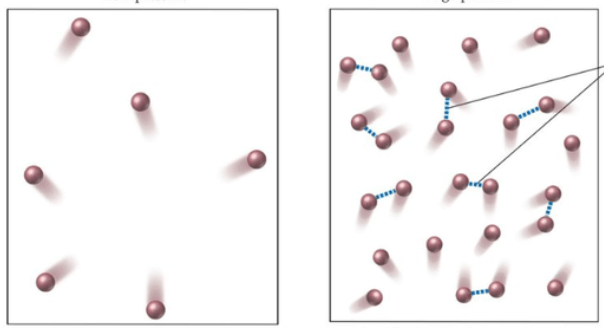
What is "pressure"?
30.0 psi is ________ than 45.0psi in kPa
What is "less"?
The height of the gas is 101 mmHg, and the height of the vacuum end is 82 mmHg. If the atmospheric pressure is 720 mmHg, then the gas pressure is __________.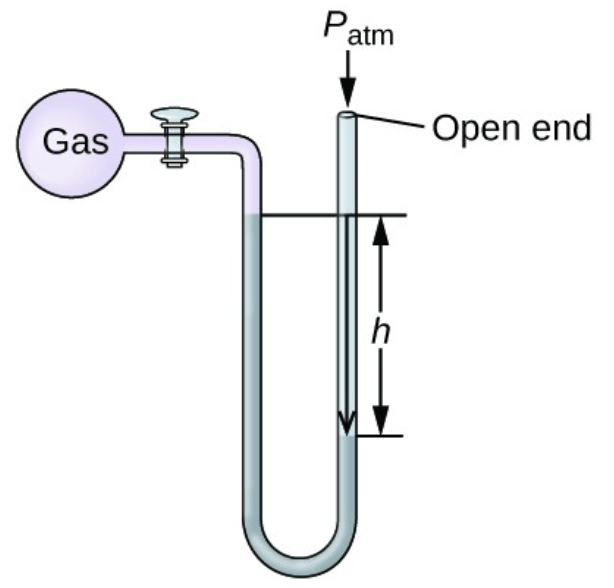
What is "711 mmHg"?
A tank is full of gases that reads 100.0 atm. Gas A is 1/2 of the total pressure, Gas B is 1/4 the total pressure and Gas C is the remaining amount. The pressure of Gas C is _______ atm.
What is "25.00" atm?
A container at 1.00 atm and 45 C was warmed to 90 C. The pressure of the container changed to _______.
What is "2.00 atm"?
The air molecules in a tire that are exerting 56.0 cmHg of pressure are also exerting _____kPa
What is "74.6 kPa"?
The height of the gas is 101 mmHg, and the height of the vacuum end is 101 mmHg. If the atmospheric pressure is 720 mmHg, then the gas pressure is __________.

What is "720 mmHg"? Or "The same"?