How is a molecule of a covalent compound different from a formula unit of an ionic compound?
A molecule is made up of two or more atoms that act as a unit.
No such discrete units exist in an ionic compound, which consists of a continuous array of ions.
According to VSEPR Theory, electron pairs
stay as far away from each other as possible
because...
electrons repel each other
Polar bonds are ________ shared and non-polar are shared ______
polar bonds are unequally shared and non-polar are shared equally
Sigma are _____ - to - _________
Sigma are end-to-end
The order of strength of the intramolecular forces is:
________ > ________ - ___________ > ________________ __________ __________
Hydrogen> dipole-dipole>London dispersion force
To name a binary molecular compound, use the following guidelines.
Write the names of the elements in the order listed in the ____________.
Use _______ appropriately to indicate the _________of each kind of __________.
If just one atom of the first element is in the formula, ____________________.
Also, the ________ at the end of a _______ is sometimes _______ when the name of the element begins with a vowel.
_____ the name of the second element with the suffix ________.
To name a binary molecular compound, use the following guidelines.
Write the names of the elements in the order listed in the formula.
Use prefixes appropriately to indicate the number of each kind of atom.
If just one atom of the first element is in the formula, omit the prefix mono- for that element.
Also, the vowel at the end of a prefix is sometimes dropped when the name of the element begins with a vowel.
End the name of the second element with the suffix -ide.
the number of hybrid orbitals =
the number of atomic orbitals mixed
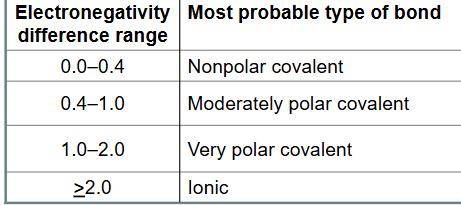
When determining the polarity of a covalent bond you must subtract the electronegativity of each element and use the difference's absolute value.
If the difference is 0 -0.4 it is _______
if it is 0.4 - 2.0 it is _______
if it is >= 2.0 it is _________.
Pi bonds are ______ - to - _________
pi is side-to-side
When a lewis structure of a covalent bond can have two different structures which are equal this called a ________ bond. And this is typically found in molecules with _______ bonds on one side and a ________ bond on the other side.
Resonance
multiple
single
4 and tetrahedral
If a molecule is symmetrical it is a polar or nonpolar moleucle?
nonpolar
Single bonds are made of how many sigma and how many pi bonds?
1 sigma bond only
Stronger intermolecular forces affect physical properties of the molecule. True or False?
True
What is the formula for Diatomic oxygen?
O2
What is the bond angle for a tetrahedral shape?
109.5
If a molecule's central atom has lone pairs, then it is a polar or nonpolar molecule?
Polar
Double bonds are made of how many sigma and how many pi bonds?
1 each
Hydrogen bonds are bonds that form between H and what three elements?
And they are between atoms in the same or different molecule?
O, F, N
different
To draw a Lewis structure for a covalently bonded molecule we must follow five steps:
1) Determine which ________ are present
2) Draw the Lewis Structure for each ________ placing the most _____________ atom in the _______.
3) Determine the number of electrons each elements needs to follow the _________ or ______ rules. Note: only ______ and _______ follow the duet rule.
4) Share an electron between two of the atoms in the molecule with a _____ bond. Where each straight/dashed line equals _____ electrons.
5) Determine if each atom is stable by following the octet or duet rule. If they do not add a ______ bond which is called a ______ bond. If it still does not make the atom stable add a _______ bond which is called a _______ bond.
To draw a Lewis structure for a covalently bonded molecule we must follow five steps:
1) Determine which __elements______ are present
2) Draw the Lewis Structure for each __atom______ placing the most ____electronegative_________ atom in the _center______.
3) Determine the number of electrons each elements needs to follow the ____octet_____ or __duet____ rules. Note: only ___Helium___ and ___Hydrogen____ follow the duet rule.
4) Share an electron between two of the atoms in the molecule with a _single____ bond. Where each straight/dashed line equals ___2__ electrons.
5) Determine if each atom is stable by following the octet or duet rule. If they do not add a __second____ bond which is called a _double_____ bond. If it still does not make the atom stable add a _third______ bond which is called a ____triple___ bond.
When we have a tetrahedral molecule that we determine to be non-polar, are the intermolecular forces dipole-dipole or London dispersion forces?
Dipole-Dipole can only happen in polar molecules so it must be London dispersion forces
If a molecule is linear, will it be a polar or nonpolar molecule?
polar
Triple bonds are made of how many sigma and how many pi bonds?
1 sigma
2 pi
The stronger the intermolecular forces then the higher the:
___________ point
__________ point
___________ tension
and the lower the
____________ pressure
