Which rheological layer of the earth exhibits plasticity and undergoes heat transfer via convection?
the asthenosphere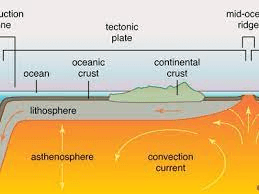
Chemical formula for quartz?
SiO2
The process of a liquid becoming a gas is known as
Evaporation (or vaporization)
Define temperature
The measure of the average kinetic energy of an object's particles.
Provide an example of radiation.
Getting sunburnt, holding your hands near a fire, sitting in a tanning bed...
Complete the definition:
A mineral is _____________.
a naturally occurring crystalline solid.
Name at least 2 units of energy!
joules, calories
What happens to the temperature of the substance as it undergoes a phase change?

The temperature stays constant during a phase change.
How would you compare the kinetic energy of particles between a gas and a solid?
The energy in a gas' particles are higher than that of a solid.
This graph shows reactants absorbing energy. What type of reaction is this?
The heat is being absorbed in the system. Therefore, this is considered as an endothermic reaction.
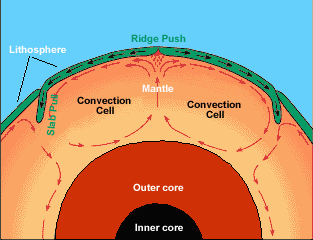
The picture above illustrates that _______ is the primary motivator of plate motion.
Convection
This is the Thingvellir fracture zone in Iceland, which is a rift valley. A rift is where the land is being pulled apart. What type of plate boundary most likely exists here?

Divergent Plate Boundaries, a rift forms, when two plates are moving away from each other.
Calculate the heat required to melt 205 g of Ice.
Given that Water's ΔHvap: 2257 J/g
Water's ΔHfus: 333.6 J/g
Values can be negative depending on the phase change
Useful Equations
Q = mHvap
Q = mHfus
68,400 J
quantity of heat needed to change the temperature of 1 g of a substance by 1 degree Celsius
specific heat (c)
Based on your knowledge of rocks, what is the name of this rock?
granite (or granitoid)
What type of plate boundary exists between the North American Plate and the Eurasian Plate?
a divergent boundary.
EARTHQUAKE!!
How much energy is required to raise 50.0 grams of iron by 55°C? The specific heat of iron is 0.444 J/g°C.
Q = mcDeltaT
= (50.0g)(0.444J/(g°C))(55°C)
= 1220 J
When 2 different temperature objects are placed in close proximity to each other, energy flows from the ______ object to the ______ one.
energy moves from the warmer object to the cooler one.
Green mineral commonly found in earth's mantle.
Olivine

Along certain parts of the Ring of Fire, the Pacific Plate is converging with the North American Plate and is being sucked into the mantle. What is this called?
subduction zone
Convection occurs in which of earth's layers? (there's more than one)
Outer core and mantle (asthenosphere)
How much energy is released when 25.0 grams of aluminum metal is cooled from 125°C to 35°C? The specific heat of aluminum is 0.900 J/g°C.
Useful Equations
Q=mcDeltaT
Answer is -2030 J
When you place a piece of ice on a piece of plastic and a piece of aluminum that are both the same temperature, you notice that the ice melts faster on the aluminum. What property of the aluminum is responsible for this phenomenon?
Aluminum's thermal conductivity is higher than plastic's!
Mr. Ly touched some magma, and it really hurt! The magma imparted 2,356 calories of energy to Mr. Ly's skin, and it raised the temperature of his skin by 45 C. Mr Ly. weighs about 80 kg... what is his specific heat in cal/g C?
.0006544 cal /g C