Whose principle is about 'opposing the change'?
Le Chatelier's
What is the difference between a strong acid and a weak acid?
A strong acid will fully ionise and a weak acid will partially ionise
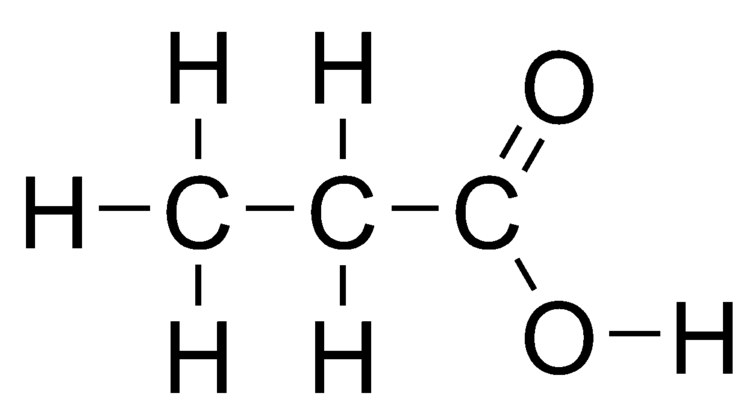
Draw propanoic acid
Name an anion (negative ion) that will always be soluble
Nitrate (NO3-)
What is molL-1 a unit of?
Concentration
Which regional city is approximately 60km from Orange?
Bathurst
Who plays 'Venom'
Tom Hardy
Complete:
K = Concentrations of _______ over the concentration of the _____
products over reactants
Name the strong acids
Hydrochloric Acid
Nitric Acid
Sulfuric Acid
Name the following compound:
CH3CH2CH(CH3)CH3
2-methyl butane
If a UV-Visible spectrum shows a peak at the wavelength of red light, what colour could the substance be?
Green-Blue
What is the formula of the Carbonate ion?
CO32-
Which central west town held Japanese Prisoners of War during WWII?
Cowra
What is the name of the latest Alien movie?
Romulus
What has changed in this system at the dotted line?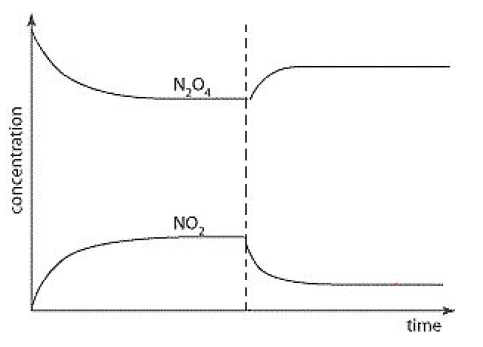
Temperature
In a titration, acid is pipetted into the conical flask, with the base going into the burette. What should the burette be rinsed in before starting this titration?
The base
Some acidified potassium permanganate is added to propan-1-ol. Name the reaction that occurs and the two possible products.
Oxidation. Propanal or Propanoic Acid.
How many carbon environments would the following compound have?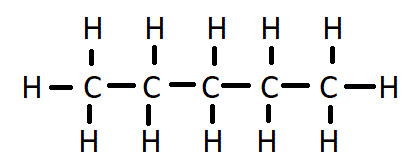
3
What colour flame would the Barium ion give?
Apple Green
Which town starting with M is about halfway between Orange and Parkes?
Manildra
Who voices Optimus Prime in the upcoming Transformers movie?
Chris Hemsworth
What is the equilibrium expression for the solubility product constant (Ksp) for Calcium Phosphate (Ca3(PO4)2)
Ksp = [Ca2+]3[PO43-]2
Acid X is a diprotic acid. It is used to neutralise some sodium hydroxide. If there are 0.2 moles of sodium hydroxide, how many moles of acid X are needed to neutralise it?
Name this compound, and also name the polymer it would form.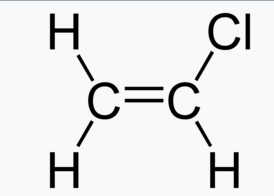
Chloroethene or vinyl chlorode. Makes polyvinylchloride (PVC)
Use a data sheet to give a possible name to the compound with the following IR spectrum, knowing it has a molecular formula of C3H6O2 and does not react with sodium carbonate.
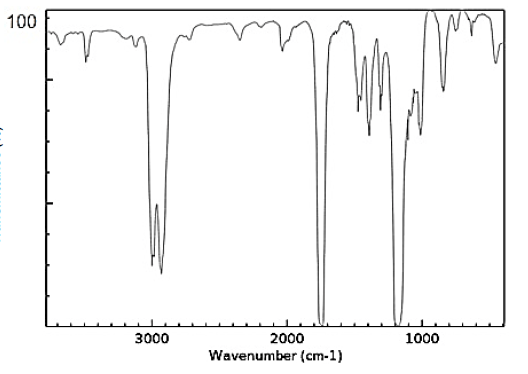
It is an ester.
methyl ethanoate or ethyl methanoate
mg/L is equivalent to what common unit?
ppm
Where in the central west would you find the Big Billy Can?
Trangie
Who played the role of the Mattel CEO in the Barbie movie?
Will Ferrell
Methane reacting with oxygen will not form an equilibrium system. Justify the spontaneous nature of this reaction by referring to Gibbs Free Energy
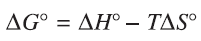
To be spontaneous Gibbs Free Energy must be negative. In the combustion reaction, enthalpy change is negative and entropy change is positive. This will guarantee that Gibbs Free Energy is negative.
A student has 0.1mol/L HCl and 0.1mol/L Acetic Acid (CH3COOH). They are going to neutralise both acids with some sodium hydroxide. Which acid will need the most sodium hydroxide to neutralise it, or will they both need the same amount?
They will both need the same amount.
Describe the steps needed to get butanone from but-2-ene
Addition Reaction using HCl. This will make 2-chlorobutane. Substitution reaction using sodium hydroxide. This will make butan-2-ol. Oxidation reaction with acidified permanganate ions or dichromate ions, this will make butanone.
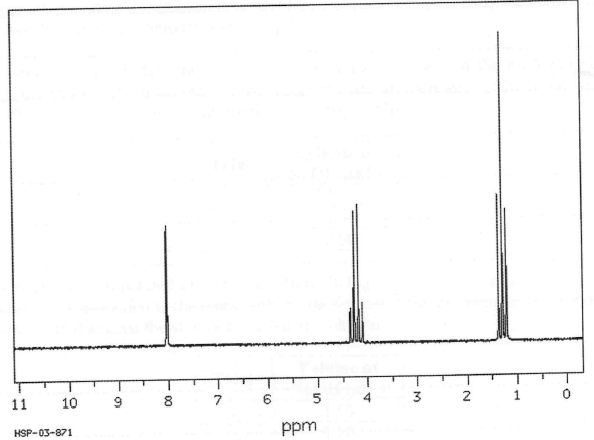 The H-NMR shows a singlet (8ppm), quartet (4.2ppm) and a triplet (1.4ppm). Knowing the substance has a molecular formula of C3H6O2 and it does not react with sodium carbonate, what is the substance?
The H-NMR shows a singlet (8ppm), quartet (4.2ppm) and a triplet (1.4ppm). Knowing the substance has a molecular formula of C3H6O2 and it does not react with sodium carbonate, what is the substance?
Ethyl methanoate
What are some tests you could conduct to show that you have a sample of calcium carbonate?
Add acid - it would react with the carbonate
Do a flame test - it would be brick red for calcium
What highways cross each other in Dubbo?
Mitchell and Newell
How many Oscars did Oppenheimer win?
7, 8, 10, 11, 12 or 13
13