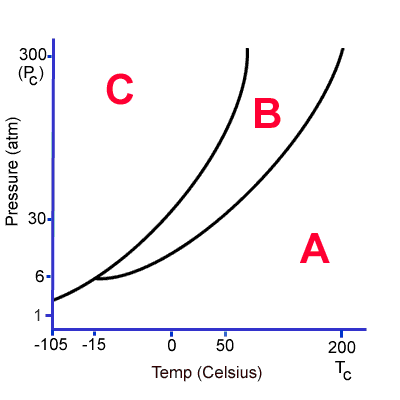
At 0C and 100 atm, what state of matter is this substance? How could I change it?
It is a solid.
I could change it to a liquid by increasing temperature. If I continued to increase temperature it would change into a gas.
I could lower pressure and it would become a gas.
List the three intermolecular forces in order of weakest to strongest.
Dispersion forces -> Dipole-Dipole -> Hydrogen Bonds
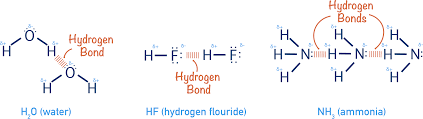
What type of intermolecular force holds two different water molecules together?
Hydrogen bonds!

What do the A, B and C represent?
A = gas
B = liquid
C = solid
What types of covalent molecules can form hydrogen bonds? Polar or nonpolar?
Polar!
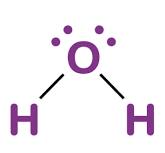
What type of bond holds oxygen to hydrogen in a water molecule?
Covalent Bonds!

What state of matter is this substance at 1 atm of pressure (below 200C)?
Gas!
Why is a hydrogen bond not an actual bond?
Because it is an intermolecular force between two different molecules. Intermolecular forces are much weaker than ionic or covalent bonds.
Draw a Lewis Dot structure for water.
Draw a phase diagram for a fake substance. Use degrees Celsius for your temperature and atmosphere for your pressure. Label the following: solid, liquid, gas, triple point, critical point.
Answers will vary
How are hydrogen bonds and dipole-dipole interactions different?
Hydrogen bonds are dipole-dipole interactions, but they also require a hydrogen bonded to a very electronegative atom being attracted to a lone electron pair on another molecule.
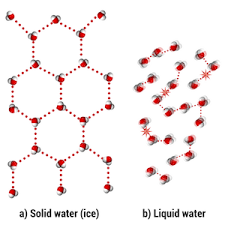
Why does solid water float in liquid water? Draw a picture to explain your ideas. (90 seconds)
Solid water floats in liquid water because when water freezes, it's hydrogen bonds connect in a 6 sided shape. This causes the water to expand, lowering the density and allowing ice to float.
Draw a phase diagram for water (use what you know about water at 1 atm of pressure). Use degrees Celsius for your temperature and atmosphere for your pressure. Label the following: solid, liquid, gas, triple point, critical point.
Answers will vary.
Draw a hydrogen bond with ALL of the details you possible can. (partial credit possible)
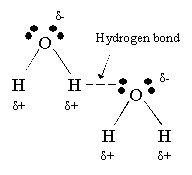
Why does water have a stronger hydrogen bonds than Ammonia (NH3)?
Oxygen is more electronegative than Nitrogen, meaning the charge difference is greater. Also, water can form two hydrogen bonds per molecules, and ammonia can only form one. This means water has a stronger hydrogen bond.