The green spheres represent sodium and the pink sphere represents chlorine. What is the atomic composition of this model?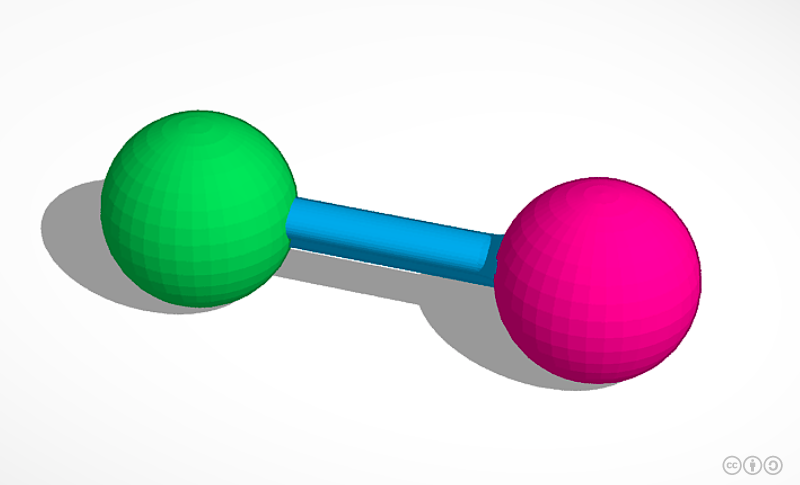
1 atom of sodium and 1 atom of chlorine.
A water molecule in the gas phase is ________ as a water molecule in the liquid phase.
the same
If a substance is made up of only one type of atom, the substance must be....
an element
T/F? A water molecule in the gas phase is the same as a water molecule in the solid phase
True
This model represents a change that results from adding ________ to a substance in a jar.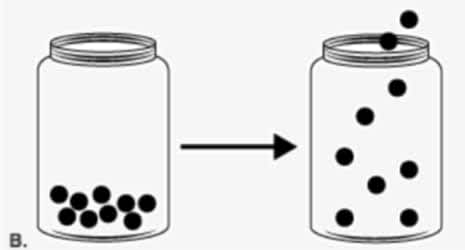
Thermal Energy or Heat
The white spheres represent hydrogen and the red sphere represents oxygen. What is the atomic composition of this model?
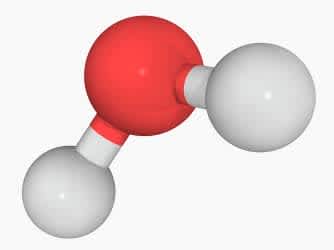
Two atoms of hydrogen and one atom of oxygen.
What state of matter is this?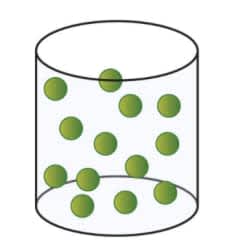
Gas; gaseous water.
How many atoms are elements made of?
One.
T/F? A pot of boiling water is covered by a lid. Water droplets begin to form on the inside of the lid because steam combined with the air to wet the inside of the lid.
False. It's because steam cools and water molecules moved closer together.
How can solid candle wax turn into a liquid?
The wax molecules are more loosely connected to each other.
The white spheres represent hydrogen and the red spheres represents nitrogen. What is the atomic composition of this model?
1 atom of nitrogen and 3 atoms of hydrogen
Is water an element or a compound?
A compound; H2O.
How many atoms of oxygen are in CO2?
Two.
T/F? If you have two metal strips and you want to see if they're the same elements, or not, you can compare their shapes.
False. The shape of a metal is not specific to it; it varies.
What is NOT made up of atoms?
Heat
The yellow spheres represent sulfur and the red spheres represents oxygen. What is the atomic composition of this model?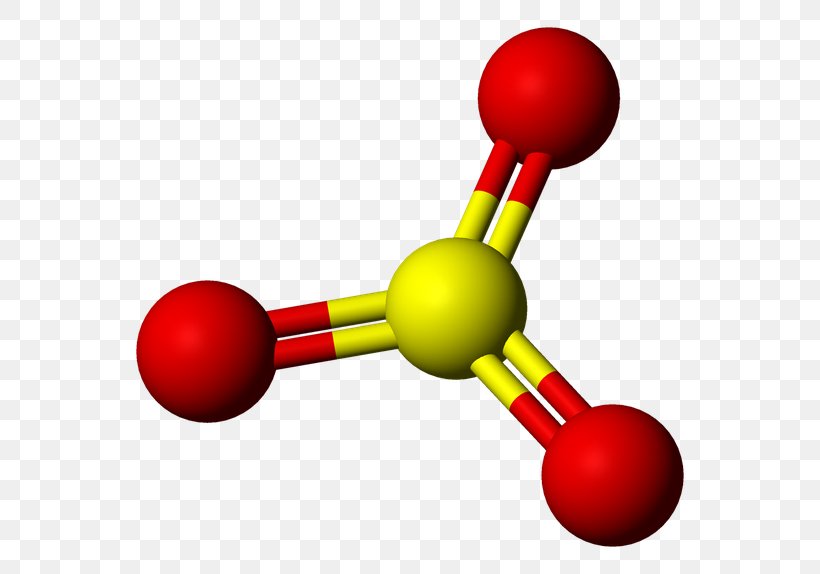
1 atom of sulfur and 3 atoms of oxygen.
What happens to the water molecules when water at 24°C is cooled to 0°C and freezes?
The molecules move more slowly.
Carbon dioxide is made of carbon and oxygen so it is a _______.
Compound
False. Matter does not have color.
Your class is studying metallic elements. Your teacher gives you two metal strips. You need to determine if they are the same element or not. What are the three different properties that would help you determine whether the strips are the same?
color
hardness (malleability)
shape
melting point
1. Color
2. Hardness (Malleability)
4. Melting Point
The black spheres represent nitrogen atoms, and the white spheres represent hydrogen atoms. Based on the model, what is the atomic composition of hydrazine?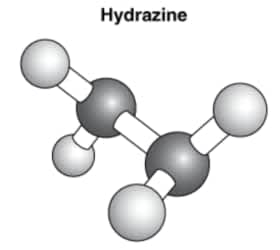
2 atoms of nitrogen and 4 atoms of hydrogen
A pot of water is placed on a hot stove. Small bubbles begin to appear at the bottom of the pot. The bubbles rise to the surface of the water and seem to pop or disappear. What are the bubbles made of?
Gaseous Water
Name an element.
Ex: Gold, Aluminum, Neon, Potassium, etc.
T/F? This model represents adding thermal energy.
True.
If temperature is rising in a pot of water, what starts to happen to molecules?
The molecules begin to move faster and spread out more.