What are the charges of Protons and Electrons?
Protons are Positive, Electrons are Negative
This state of matter has particles packed closely together in a fixed arrangement, giving it a definite shape and volume
SOLIDS
The number of protons OR electrons
This property of matter is calculated by dividing mass by volume.
Density
What is the charge of Neutrons?
Neutrons are NEUTRAL - NO CHARGE
What two particles are found in the NUCLEUS of an atom?
Protons and Neutrons
This state of matter has a definite volume but takes the shape of its container because its particles can slide past one another.
LIQUIDS
What does the atomic mass tell us about an element?
It is number of protons and neutrons found in an atom's nucleus.
Density is a measure of how much of this is packed into a given volume of a substance.
Mass
What particle is found orbiting the center mass of the atom in orbitals?
Electrons
Where is all the mass of an atom found?
In the NUCLEUS
This state of matter has no definite shape or volume because its particles move freely and spread out to fill any container.
GASES
What is the primary way elements are organized (in numerical order) on the Periodic Table?
By the Atomic Number
If an object has a density greater than water, it will do this when placed in water.
SINK
How many electrons can fit on each orbital level? (First, Second, Third, and Fourth)
First = 2, Second = 8, Third = 18, Fourth = 32
What is the name of an atom that has gained or lost an electron?
An ION
This high-energy state of matter is made of charged particles and is found in stars, lightning, and neon signs
PLASMA
In the periodic table, which direction do periods run in? Which direction do groups go?
Periods run horizontally (left to right), Groups go vertically (up and down).
If an object floats on oil but sinks in water, the object’s density is _____ than oil and _____ than water
greater than oil, less than water
What element is this?
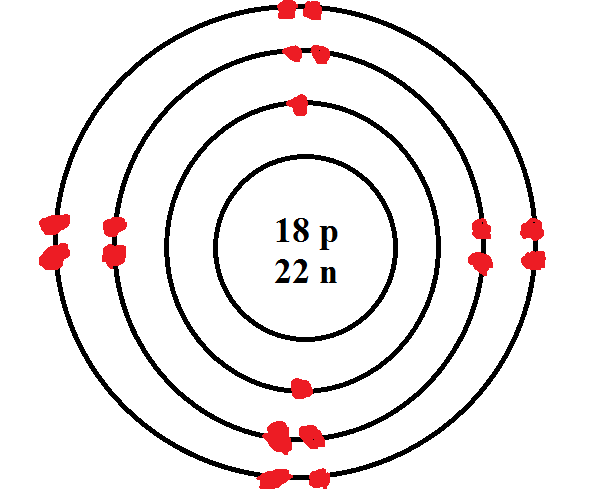
Argon!
What is the name for the electrons in the outermost shell of an atom that determine how it bonds with other atoms.
Valence Electrons
Which theory states that all matter is made of particles that are constantly in motion?
Kinetic Theory of Matter
What are the three categories of periodic elements?
Metals, Metalloids (Semi-conductors), and Nonmetals
A liquid has a mass of 250 g and a volume of 200 mL. What is its density in g/mL?
Density = 1.25 g/mL
What element is this based off the number of electrons?![]()
Silicon