What is the chemical formula for the compound formed from potassium and oxygen?
K2O
Give the chemical formula for dinitrogen pentoxide
N2O5
What are the 3 components of collision theory?
1. Molecules must collide to react
2. Molecules must collide with sufficient energy to react.
3. Molecules must collide at the correct orientation to react.
How many moles are in 25.7 g of BF3?
0.379 mol BF3
What is transmutation?
A change in the number of protons or neutrons in the nucleus.
What are 2 differences between ionic and covalent bonds?
Ionic- metal + nonmetal, metal gives electrons to nonmetal
Covalent- nonmetal + nonmetal, atoms share electrons
Name the following compounds:
Na3P
CaCl2
sodium phosphide, calcium chloride
What are 3 things that will speed up the rate of a reaction? Why?
1. increase temperature (more and harder collisions)
2. increase surface area (more collisions)
3. increase concentration (more collisions)
2 BrCl3 --> 3 Cl2 + Br2
If 3.54 moles of BrCl3 reacts according to the equation, how many moles of Cl2 will be formed? How many moles of Br2 will be formed?
5.31 mol Cl2
1.77 mol Br2
What is radioactive decay? Which elements are radioactive?
The spontaneous disintegration of a nucleus to form a smaller nucleus. Transuranium elements.
Draw the Lewis structure and give the VSEPR shape for the following:
- CHCl3
- BF3
- NF3

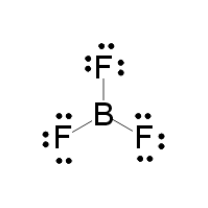
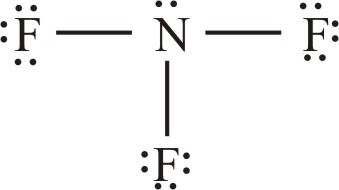
tetrahedral, trigonal planar, trigonal pyramidal
Give the prefixes used for naming covalent compounds
Draw and label the energy diagrams for an exothermic and endothermic reaction.
Include: axis labels, activation energy, reactants, products, and the overall energy change

__AgNO3 + __BaCl2 --> __AgCl + __Ba(NO3)2
If 410.8 grams of barium nitrate are produced how many grams of silver nitrate were reacted?
534.1 g AgNO3
What are the 3 types of particles that can be emitted during radioactive decay? Write your answers in nuclear symbol notation.
42He (alpha particle), 0-1e (beta particle), 01B (positron)
Ascorbic acid (vitamin C) is 40.92% C, 4.58% H and 54.50% O by mass, and has a molar mass of 176.1 g/mol. What are the empirical and molecular formulas of ascorbic acid?
Empirical: C3H4O3
Moleulcar: C6H8O6
Name the following acids:
H2Se
H2SO4
H3PO3
Hydroselenic acid, sulfuric acid, phosphorous acid
Predict the products and give the reaction type for the following:
Na(s) + MgCl2(aq) -->
Al(s) + O2(g) -->
C2H6(g) + O2(g) -->
Mg(s) + NaCl(aq), single replacement
Al2O3(s), synthesis
CO2 + H2O, combustion
2 Mg + O2 --> 2 MgO
What is the limiting reactant if 2.2 g of Mg is reacted with 4.5 L of oxygen at STP? How many grams of the product will form?
Mg is the limiting reactant, 3.6 g MgO will be formed.
What is half-life? The half-life of plutonium-239 is 24,300 years. If a nuclear bomb released 8 kg of this isotope, how many years would pass before the amount is reduced to 1 kg?
The amount of time it takes for half of a substance to decay. 72,900 years.
Write the chemical sentence showing the formation of aluminum sulfide.

Provide the name or formula for the following:
chromium (II) perchlorate
iron (III) phosphate
Cu2C2O4
Cr(ClO4)2
FePO4
copper (I) oxalate
Write the molecular, complete ionic, and net ionic equations for the reaction of barium nitrate and sodium sulfate
Ba(NO3)2(aq)+Na2SO4(aq) --> BaSO4(s) + 2NaNO3(aq)
Ba2+ + 2NO3- + 2Na+ + SO42- --> BaSO4 + 2Na+ + 2NO3-
Ba2+ + SO42- --> BaSO4
Sodium chloride reacts with lead (II) nitrate to form lead (II) chloride and sodium nitrate. Write the balanced equation.
How many grams of lead (II) chloride are produced from the reaction of 15.3 g of sodium chloride and 60.8 g of lead (II) nitrate? What is the limiting reactant? How much excess is left over?
2 NaCl + Pb(NO3)2 --> 2 NaNO3 + PbCl2
36.4 g PbCl2 are produced, NaCl is the limiting reactant, 17.4 g Pb(NO3)2 are left over.
Write the equation for the alpha decay of radium-226.
Write the equation for the beta decay of nickel-63.
22688Ra --> 42He + 22286Rn
6328Ni --> 0-1e + 6329Cu