The word(s) that IMF stands for.
intermolecular forces
The IMF bond with the weakest IMF?
London dispersion force
Which formula represents an ionic compound?
A) H2O
B) Cl2O
C) HBr
D) KCl
D) KCl
What two things can you never eat for breakfast?
Lunch and Dinner
Who of the original Avengers died by the end of the Infinity Wars?
Black Widow, Ironman
The definition for intermolecular force?
the attraction between different substances or compounds
When substances have a high melting or boiling point, that means their intermolecular forces are stronger or weaker?
stronger
In the H–Cl bond, the two bonding electrons are?
A) closer to the chlorine atom
B) closer to the hydrogen atom
C) totally removed from the hydrogen atom
D) totally removed from the chlorine atom
A) closer to the chlorine atom
Which word in the dictionary is spelled incorrectly?
Incorrectly
What is the name of the fairy in Peter Pan?
Tinkerbell
the intermolecular attraction resulting from temporary attraction of atoms
London Dispersion Force
Hydrogen bonding is a type of?
A) strong covalent bond
B) weak ionic bond
C) strong intermolecular force
D) weak intramolecular force
C) strong intermolecular force
Which would be less polar? HF or HBr
HBr
In the Marvel movies, which movie would you watch first, in chronological order?
Captain America: The First Avenger
What time is shown?
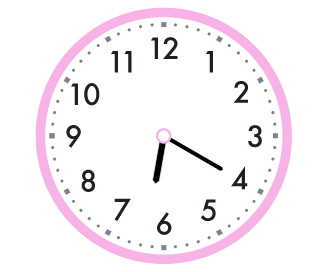
6:20
Intermolecular forces are not one of these:
London dispersion
ionic bonding
dipole dipole
hydrogen bonding
ionic bonding
Weakest IMF to the Strongest IMF's?
dispersion, dipole-dipole, hydrogen bonding
What is the bond polarity of the substance?
NH3
NH3 = polar covalent
Is energy being absorbed or released?
Br2 + energy → Br + Br
Energy is absorbed so that Br bonds are broken.
Is 6/10 and 36/60 equal fractions?
Yes
The basic types of intramolecular forces?
ionic bond, covalent bond, metallic bond
Which has stronger intermolecular forces? H2O or CO2
H2O since it has hydrogen bonding but CO2 does not.
Which statement explains the low boiling point of hydrogen, H2 compared to HCl?
A) H2 has strong intermolecular forces.
B) H2 has weak intermolecular forces.
B) H2 has weak intermolecular forces.
State the approximate atomic mass of dihydrogen monoxide?
What is the McDonald's mascot?
Clown/Ronald McDonald