IMF stands for...
Intermolecular forces
The three types of subatomic particles in an atom.
What are protons, neutrons, and electrons?
What is an isotope?
Atoms with the same number of protons but different number of neutrons.
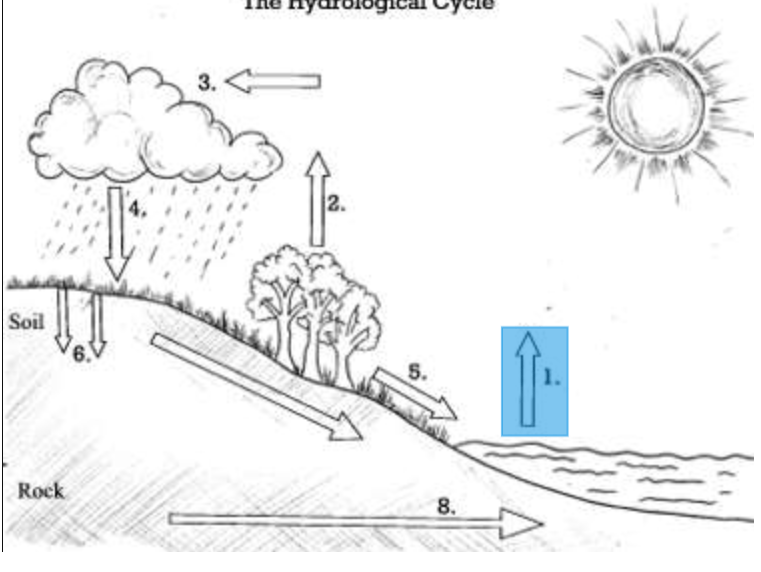
Identify the process: Water released by plants into the atmosphere
Transpiration
In a chemical reaction the substances to the right of the arrow are called this/these?
Products
What is Ionic Bonding?
It is the complete transfer of valence electrons. It is also force of attraction between oppositely charged ions.
Intermolecular forces are __________ molecules
Between
Where can you find the atomic number? Is it the larger or smaller number.
Periodic table, on top of atomic symbol.
There are two ways to notate an isotopes. Show both notations for carbon-13.
C-13 or 13C
Identify the process: Water changes from liquid to gas by absorbing energy from the sun.
Evaporation
A substance that enters a chemical reaction is called this.
a reactant
What is Covalent bonding?
Covalent bonding results from the attraction between the nuclei of atoms where electrons are shared.
What are the 3 basic types of intermolecular forces.
London dispersion forces, dipole-dipole interactions, and hydrogen bonds
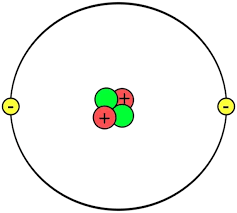 Given the following image, what element is this?
Given the following image, what element is this?
Helium
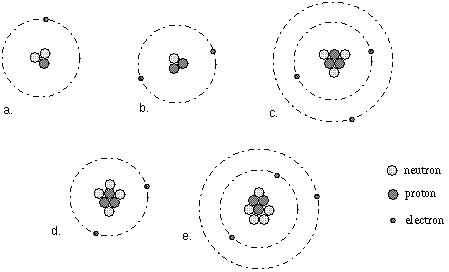
Which of the following are isotopes of one another and why?
C, D and E are isotopes because all of them have the same number of protons (3). They are all isotopes of Lithium.
What is the main source of energy for the Water Cycle?
THE SUN
When balancing an equation, these numbers CAN be changed.
a coefficient
Is Na and Cl a Ionic or covalent bond?
It's an ionic bond.
The strongest type of IMF.
What is an hydrogen bond?
What is an ion?
A charged atom. An atoms that's gained or lost electrons.
How many neutrons does the isotope Neon-22 have?
Show your work on the board
Neon-22
22 (mass) - 10 (atomic #) = 12 neutrons
Chemicals react with the minerals in rocks to change and break down the rocks is called
Chemical Weathering
In the formula 5CaSO4,how many oxygen atoms do we have?
20
Is hydrogen and hydrogen and ionic or a covalent bond?
It's a covalent bond.
The stronger the IMFs, the _____ the boiling and melting points.
Higher
What are the two types of ions? What are their charges?
Cation-positive and anion-negative.
Based on the picture below, identify the element and the number of valence electrons it contains.
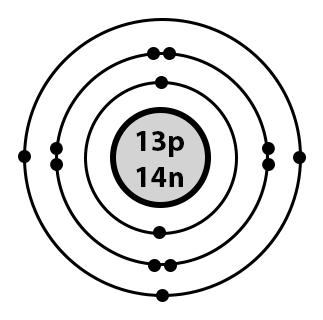
Aluminum; 3 valence electrons
Rocks broken apart by water, wind and ice.
Physical Weathering
How many Aluminum atoms do we have in the products?
Al + O2 → Al2O3
2
What type of bond in Carbon dioxide?
It's a covalent bond.
What type of IMF is present? Name ALL
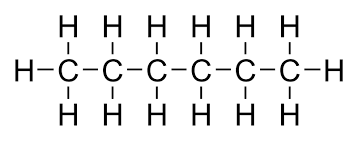
London Dispersion Force (LDF)
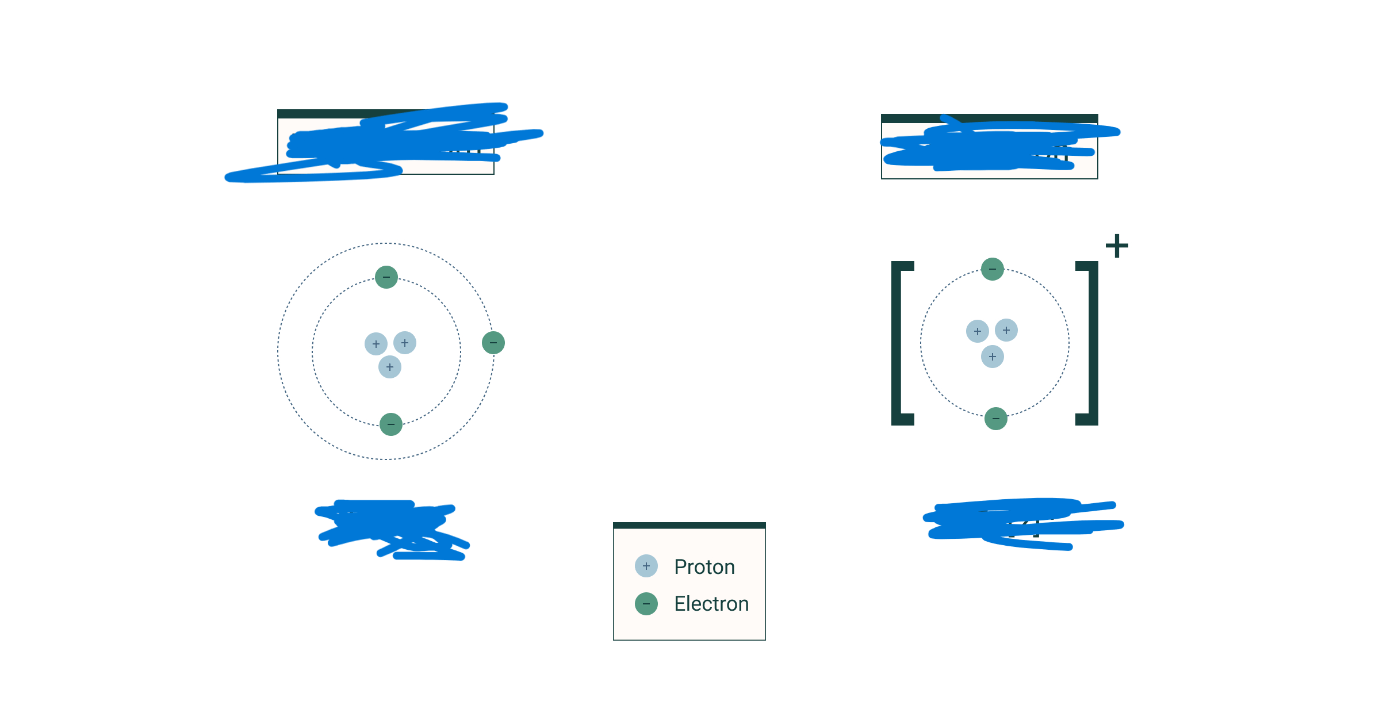
Given this image, write element name and correct symbol.
Lithium. Li+1
Draw the Bohr model for Magnesium.

What can happen when water gets inside a rock and freezes?
The rock can break/crack
Is this a balanced or not balanced equation?
H2S + NaCl → HCl + Na2S
Not balanced
Is calcium chloride an ionic or covalent bond?
It's an ionic bond.
What type of IMF is present? Name ALL
LDF
Dipole-dipole
Hydrogen Bond
An element has 15 protons and 18 electron, is this an ion? If so, what type? Correct symbol?
Ion. Anion. P-3
A chemistry student draws the Bohr model for Carbon. The class knows carbon has 6 electrons, when neutral. The student places 4 electrons on energy level 1 and 2 electrons on energy level two. Is their Bohr model draw correctly? Why or why not? Draw it out on the board
Energy level 1 can only carry 2 electrons and energy level 2 can carry up to 8 electrons.
Precipitation
Is this equation a balanced or not balanced chemical equation? If not balanced, give balanced equation.
P4 + O2 → P4O6
Not balanced
P4 + 3O2 → P4O6
What bond is a CH4 molecule?
Covalent bonding.