The state of matter with particles that are close together and vibrate slowly in place.
What is a solid?
The equation and units for density
What is d = m ÷ v and g/cm3 or g/mL
Aluminum has a mass of 27 g and a volume of 10 cm3. Calculate the density and include the units. Hint: d = m ÷ v
What is 2.7 g/cm3
One of these objects is the least dense.
What is C?
A physical change is this while a chemical change is not this.
What is reversible?
The state of matter has a definite volume but not a definite shape.
What is a liquid?
The difference between mass and weight
What is gravity
Carbon monoxide gas has a mass of 0.196 g and occupies a volume of 100 mL. Calculate the density and use the correct units. Hint: d = m ÷ v
What is 0.00196 g/mL
What is thermal conduction?
Burning, rusting, changing color, and gas formation are all key indicators of what type of change?
What is a chemical change?
The scientific theory that all matter is made of atoms.
What is the atomic theory?
The equation to calculate the volume of a regular solid and units
What is L x W x H and cm3
A block of aluminum occupies a volume of 15.0 mL and has a mass of 40.5 g. Calculate the density and include the units. Hint: d = m ÷ v
What is 2.70 g/mL
True or False: the boiling and melting points of water will stay the same no matter how much water there is
What is true?
When you add this to a reaction, the reaction rate will increase.
What is thermal energy?
The state of matter that sound travels the slowest but light travels the fastest.
What is a gas?
The equation to calculate volume using the water displacement method
What is measurement after - measurement before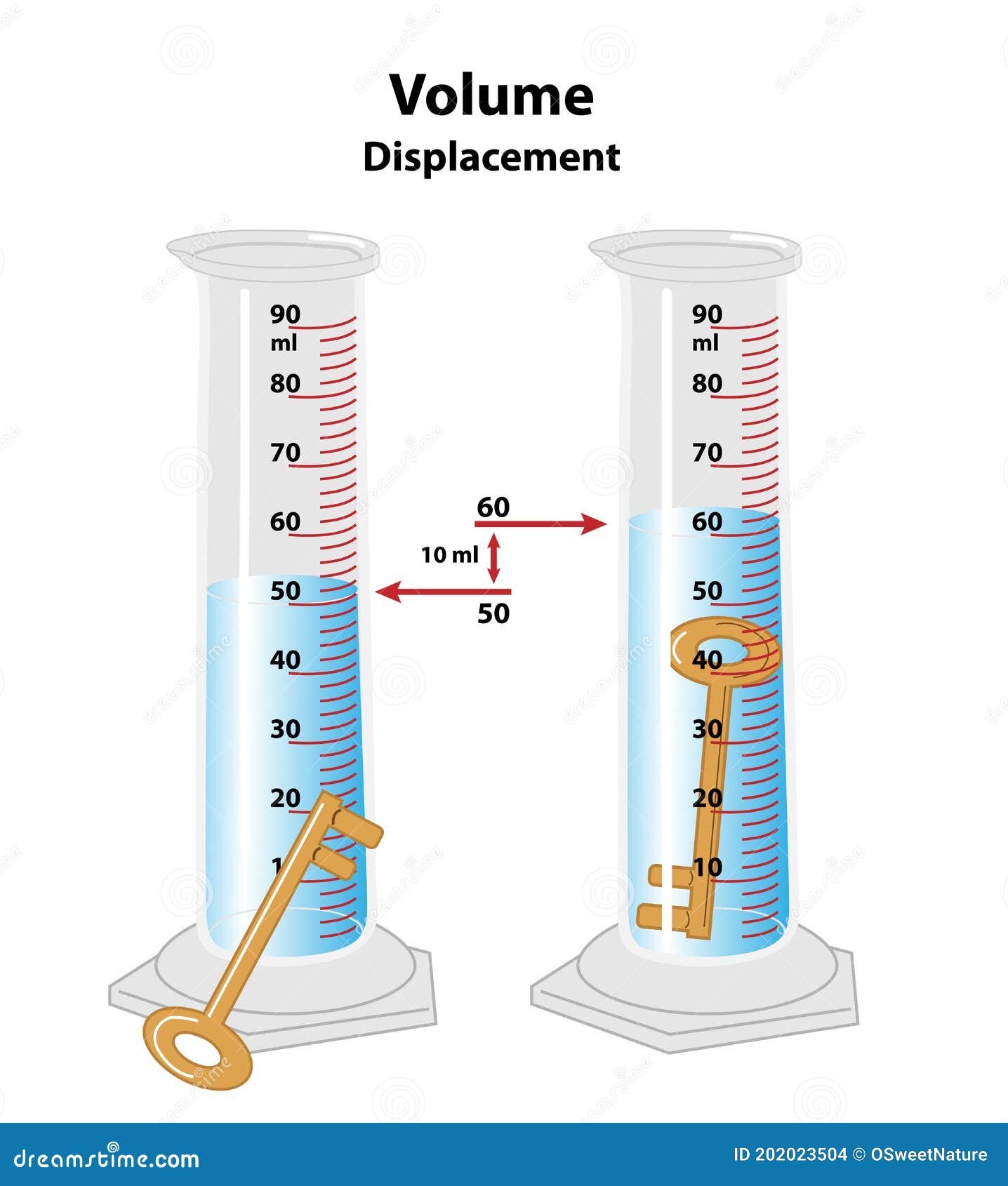
A block of wood is 3 cm on each side and has a mass of 27 g. Calculate the volume and density of the block and include units. Hint: L x W x H to find volume and d = m ÷ v
What is 27 cm3 and 1 g/cm3
True or false: You can add an unlimited amount of a solute to a solvent and it will always dissolve.
What is false?
Baking soda and vinegar are measured and have a total mass of 25 grams. When they are combined, the solution bubbles up and releases a gas. The total mass afterwards is 18 grams. What happened to the other 7 grams?
What is it was released as a gas?
A pencil looks bent when it is put into a cup of water. This is due to...
What is refraction?
An object with a higher density than water will do what
What is sink?
A graduated cylinder has 45 mL of water in it. A rock with a mass of 12 g is placed into the cylinder and the water level went up to 61 mL. Calculate the volume and the density. Hint: measurement after - measurement before to find volume and d = m ÷ v
What is 0.75 g/mL
Different substances have unique physical properties. This can be very helpful in doing what?
What is identifying them?
When a candle is lit, the wick burns, the wax melts, the candle changes shape, and the air around the candle heats up. When does the chemical change happen?
What is the wick burning?