Which of the following are ionic compounds?
CaCl2
K2S
N2O5
CCl4
CaCl2
K2S
What is the boiling point of a substance at standard pressure?
120 C
Give the name for the following formulas of binary molecular compounds
H2O2
CCl4
Dihydrogen dioxide
Carbon tetrachloride
Which elements are the exception to the octet rule and how many electrons do they need for a full valence shell?
H and He, 2 ve
Using electronegativity, determine type of bond Li2O has.
3.5-1.0= 2.5
Ionic bond
What phase of matter would this substance be in at 200 C and 1.00 atm?
Liquid
Ionic or Molecular: which is most likely not to dissociate in water?
Molecular
Which of the following elements will form cations?
Calcium
Nitrogen
Oxygen
Potassium
Calcium
Potassium
Consider aqueous solutions of the four compounds:
NaCl C6H12O6 Li2O CH4
Which two will have a lower melting point? Explain why.
C6H12O6 and CH4
What two processes would cause a substance to transition between 4 and 3 on a phase diagram?
evaporation and condensation
Which of the following formulas represent an ionic compounds that can be made from these six elements: Al, Be, F, Mg, Li, and S?
Mg3Al2
BeS
AlLi
MgF2
MgF2
BeS
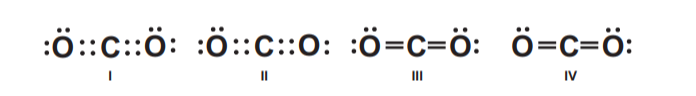
I and III
Which of the following statements about metals is false?
Metals can be drawn into wires
Metals have low melting and boiling points
Metals can be hammered into different shapes
Metals in solid form have specific crystal structures
Metals have low melting and boiling points
Approximately what temperature and pressure is the triple point at?
100 celsius and 0.75atm
Copper is a metal and has a Cu2+ ion that reacts with a nonmetal, bromine, which has an ion of Br-. Predict the ratio of copper to bromine in an ionic compound assuming the ions bond in the reaction.
1 Cu: 2 Br
Give the formula for the following binary molecular compounds
Disulfur dioxide
Nitrogen monoxide
Xenon hexaflouride
S2O2
NO
XeF6
What are the 10 prefixes we use for covalent molecules? Give both the name and the number they represent.
mono- 1
di- 2
tri- 3
tetra- 4
penta- 5
hexa- 6
hepta- 7
octa- 8
nona- 9
deca- 10
Name the processes that result in the following phase changes:
Solid to gas
Liquid to gas
Liquid to solid
Gas to liquid
Sublimation
Evaporation
Freezing
Condensation
Which of the tug-of-war figures BEST represents two bonded atoms with electronegativity differences that are less than 0.4? Explain your answer
Figure A
Give the formula for the following ionic compounds
Lead (II) nitrate
Potassium iodide
Magnesium hydroxide
Pb(NO3)2
KI
Mg(OH)2