A bond between a metal and a nonmetal
ionic bond?
Potassium loses this many valence electrons when it becomes an ion.
One
A bond between 2 or more nonmetals
covalent bond
Double Jeopardy: NO
What is nitrogen monoxide?
The type of ion formed when metals lose electrons
Cation?
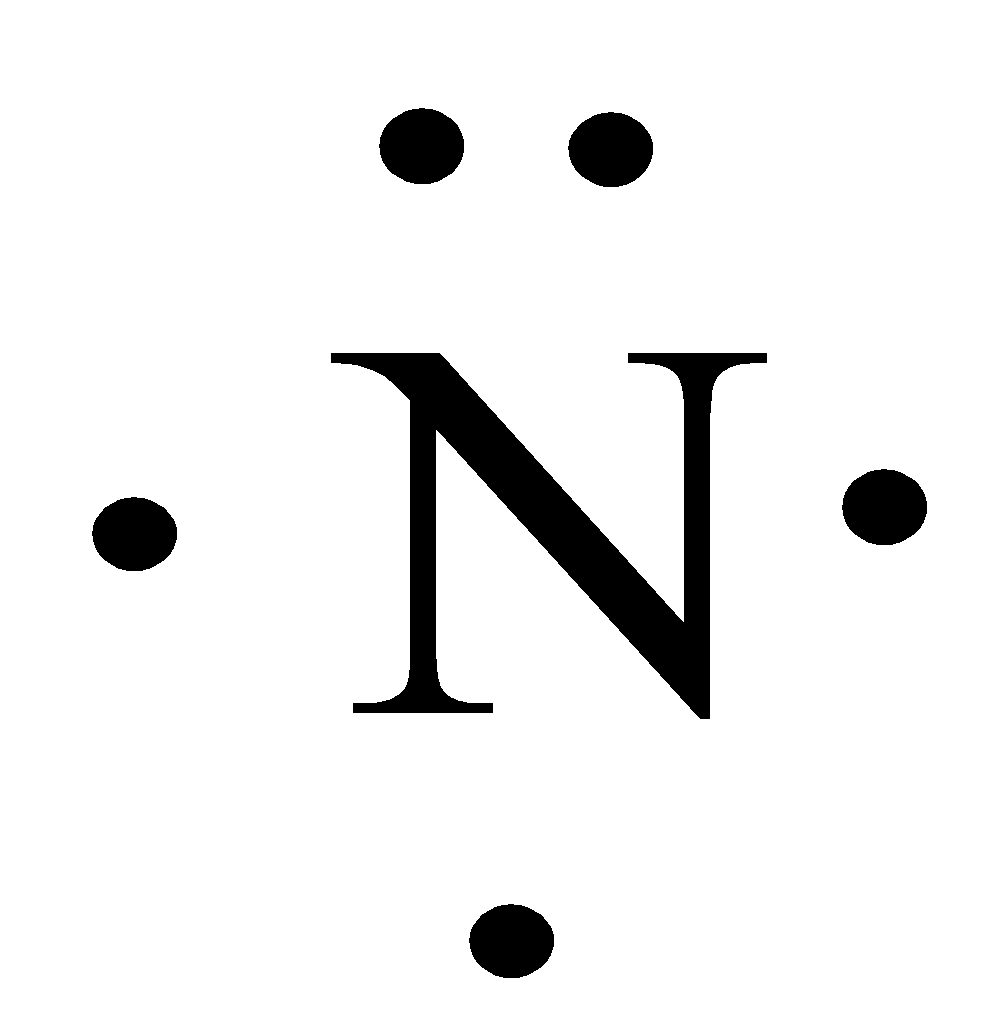
The electron dot structure for N
BaF2
What is barium fluoride?
In covalent bonds electrons are ____________.
shared
CH4
carbon tetrahydride
The type of ion formed when nonmetals gain electrons.
Anion
Oxygen has this many valence electrons
6
FeS
iron (II) sulfide
Molecules and compounds have a ______________ charge.
neutral
N2O
dinitrogen monoxide
The electrons in the highest occupied energy level of an element's atom
valence electrons?
The number of valence electrons found in He
2
NaOH
Double Jeopardy: H2 and O2 are examples of ___________ molecules.
diatomic
H2O
dihydrogen monoxide
The rule that states that in forming compounds, atoms tend to achieve a full valence shell, like those of noble gases.
The Octet Rule?
This group forms -1 ions
Group 7 or Group 17
Double Jeopardy: Pb3(PO4)4
lead (IV) phosphate?
A double bond represents a total of ______ electrons.
4
Be(OH)2
beryllium hydroxide