Write out the formula for :
Na + N
Be sure to include charges and show work
Na1+3N3-
Write out the formula for :
H + F
Be sure to include charges and show work

The dot diagram for H2 would be ?
Bonds formed by a ____ of electrons are ionic bonds
Transfer
NaCl
Ionic
Write out the formula for :
Ba + O
Be sure to include charges and show work
Ba2+O2-
Write out the formula for :
H2 + S
Be sure to include charges and show work
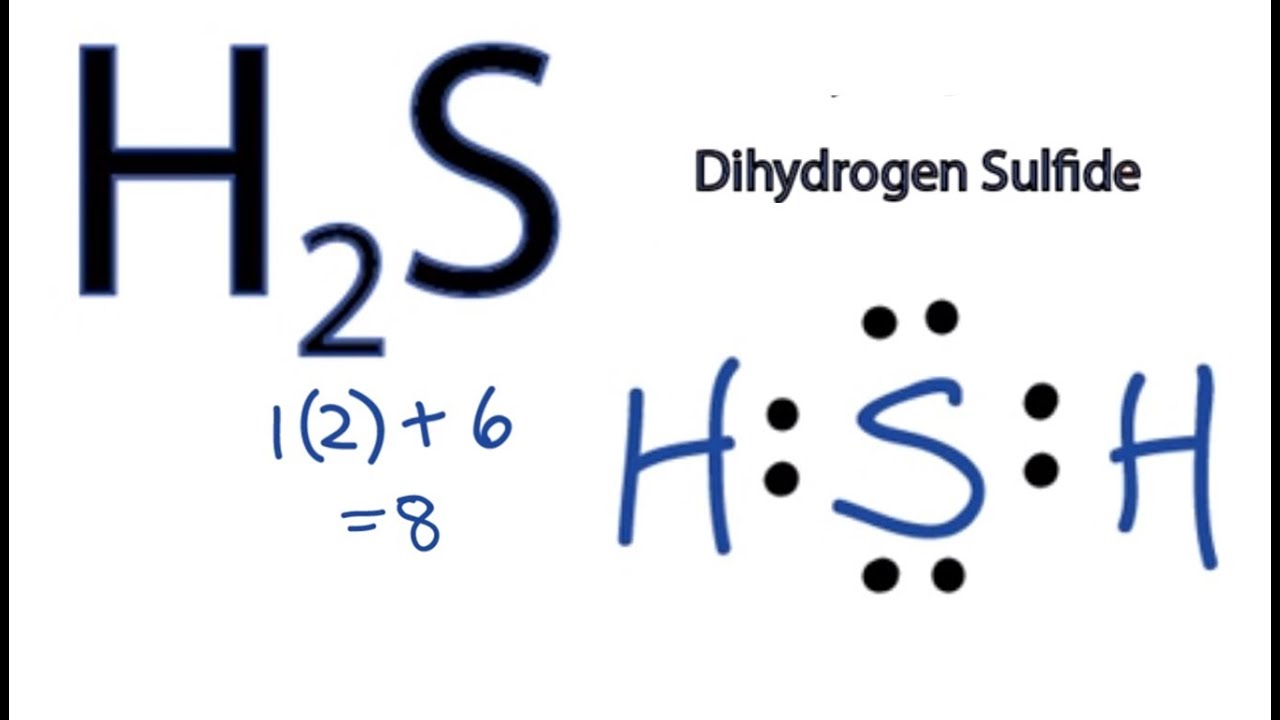
The dot diagram for BeF2 would be ?

Bond formed by a ____ of electrons are covalent bonds
Sharing
PbI
Ionic
Write out the formula for :
Al + S
Be sure to include charges and show work
Al+32S-23
Write out the formula for :
N + I3
Be sure to include charges and show work

This dot diagram represents the chemical formula______________
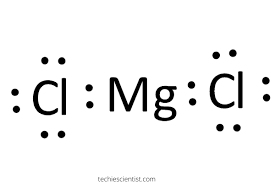
MgCl2
What two types of elements form ionic bonds and what two type of elements form covalent bonds?
metal and non mental ( ionic)
nonmetal and nonmetal (covalent)
BrCl
Covalent
Write out the formula for :
Al + Cl
Be sure to include charges and show work
Al3+Cl1-3
Write out the formula for :
C + Br4
Be sure to include charges and show work

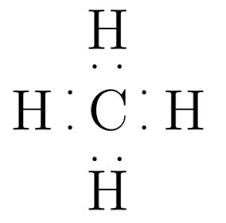
The Lewis dot diagram for CH4 would resemble
a positively charged ion
cation
AlO
Ionic
Write out the formula for :
Al + O
Be sure to include charges and show work
Al3+2-O2-3
Write out the formula for :
C2 + H2
Be sure to show work

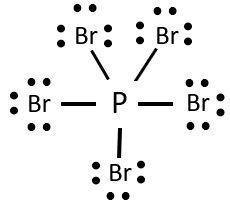
The Lewis dot diagram for PBr5 would resemble
Octet rule states that atoms tend to form bonds so each atom has ___ electrons in it's ___ shell.
8 electrons in it's valence shell
NeF
Covalent