Elements in this group have 5 valence electrons
group 5/15
This property of ionic compounds is based on the strength lattice energy, the stronger this property the higher the value of the property
melting or boiling point
KI
Potassium Iodide
Strontium chloride
SrCl2
CuCl2
NiCl2
Nickel (II) Chloride
Write the Formula and Draw the Bond
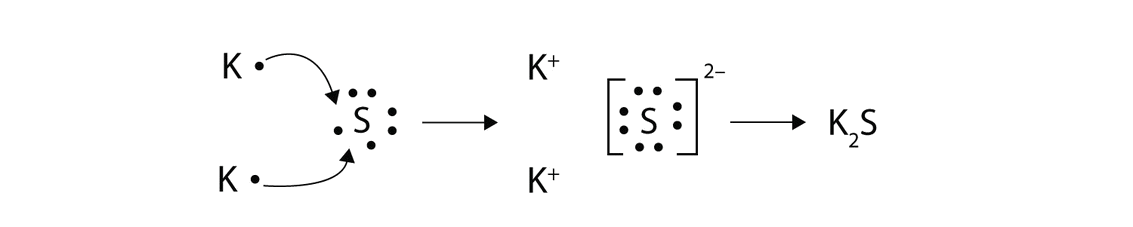
Potassium and Sulfur
The structure of an Ionic Compound is called a
Crystal Lattice
UCLA
University of California - Los Angeles

PayPal

Lana Del Ray

Tiramisu
Elements in this group have a charge of 2+
Group 2
Alkaline Earth Metals
When Ionic Compounds dissociate in water, ions are formed. Another name for Ions are ___________.
electrolyte
CaS
calcium sulfide
Potassium sulfide
K2S
Iron (III) Oxide
Fe2O3
Iron (III) Chloride
FeCl3
Write the Formula and Draw the Bond
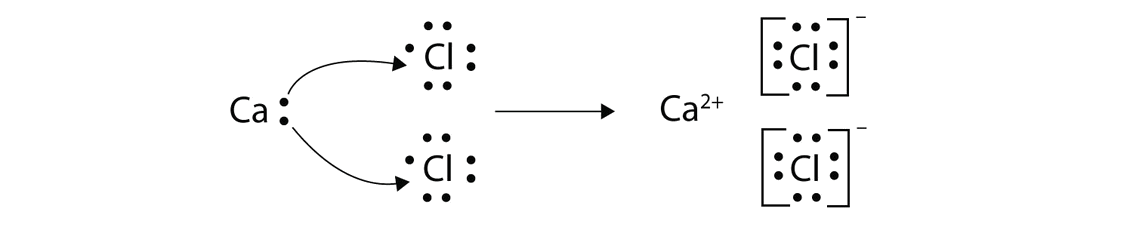
Calcium and Chlorine
The Crystal Lattice is made up of ...
Formula Units
TCU
Texas Christian University

Southwest
Tate McRae
/__opt__aboutcom__coeus__resources__content_migration__simply_recipes__uploads__2018__02__Creme-Brulee-LEAD-50a2a2b099d840ce8796c2e2793f254f.jpg)
Creme brulee
Elements in this group have 3 valence electrons
group 3/13
This describes ionic compounds when an external force is applied to a solid
Brittle
BaS
Barium Sulfide
Beryllium oxide
BeO
Titanium (IV) Oxide
TiO2
Zinc (III) Bromide
ZnBr3
Write the Formula and Draw the Bond
Magnesium and Oxygen
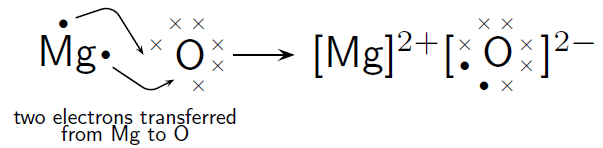
When a Formula Unit is broken up, the resulting parts are called.
Ions
Fairleigh Dickinson University

Los Angeles Lakers

Sabrina Carpetner
Shaboozey
Chappell Roan

Red Velvet Cake
Elements in this group do not form ions easily
group 8/18
Ionic Compounds can conduct electricity during which two incidents?
Dissociated in Water and Molten
CaCl2
Calcium Chloride
Aluminum Oxide
Al2O3
Silver (I) Chloride
AgCl
Vandium (V) Oxide
V2O5
Write the Formula and Draw the Bond
Sodium and Chlorine
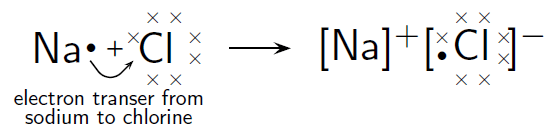
Ionic Compounds are held together, by this force of attraction.
Electrostatic Force
FAU
Florida Atlantic University

Boston Red Sox
Jack Harlow
Kendrick Lamar
The Weeknd
Drake
This group of elements forms no charge
group 8/18
Ionic Compounds form a ______________ which is a repeating pattern, while Covalent Compounds form individual _____________.
crystal lattice | Molecules
Al2O3
Aluminium Oxide
Potassium Nitride
K3N
Manganese (VII) Oxide
Mn2O7
Scandium (III) Nitride
ScN
Write the Formula and Draw the Bond
Beryllium and Bromine
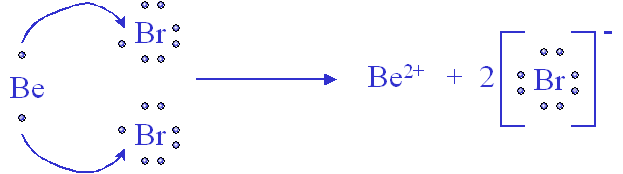
Ionic Compounds are the attraction from a __________ and _____________.
Cation and Anion
VCU
Virginia Commonwealth University

Gucci
Stevie Nicks
Shakira
Bob Dylan

Baklava