name this
create the compound
vocabulary
misc
100
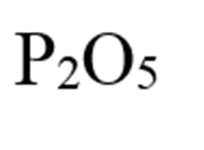
What is diphosporous pentoxide?
100
The Lewis dot or electron dot diagram for aluminum has how many dots around the element symbol?
What is 3 dots to show the number of valence electrons?
 Bonus question:
For group A elements, what number do we look for to identify the number of valence electrons any group A element has?
Bonus bonus question:
How many valence electrons does helium have?
Bonus question:
For group A elements, what number do we look for to identify the number of valence electrons any group A element has?
Bonus bonus question:
How many valence electrons does helium have?
 Bonus question:
For group A elements, what number do we look for to identify the number of valence electrons any group A element has?
Bonus bonus question:
How many valence electrons does helium have?
Bonus question:
For group A elements, what number do we look for to identify the number of valence electrons any group A element has?
Bonus bonus question:
How many valence electrons does helium have?100
These elements are generally non-reactive and typically do not form chemical bonds with other elements.
What are noble gases?
200
NaOH
What is sodium hydroxide?
200
Chemical formula created when aluminum and oxygen bond.
What is  ?
Make certain you can name this and identify if it is ionic or covalent!
?
Make certain you can name this and identify if it is ionic or covalent!
 ?
Make certain you can name this and identify if it is ionic or covalent!
?
Make certain you can name this and identify if it is ionic or covalent!200
When an atom will not react with another atom to form a chemical bond we say that it has a ________________.
What is stable electron configuration?
300
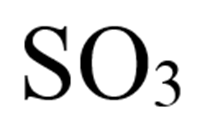
What is sulfur trioxide?
Bonus Question:
Describe the number and type of covalent bonds in the compound. Here is the structural model but you should know how to draw them:


300
This is the chemical formula for sulfur hexafluoride.
What is  ?
?
 ?
?
300
This term is used to define how well an atom can attract an electron to it (related to the amount of energy required to remove an electron from the outer shell).
What is electronegativity?
400
Water is an example of a ___________ covalent compound.
What is polar?
400
What is the chemical formula for Calcium Nitrate?
What is 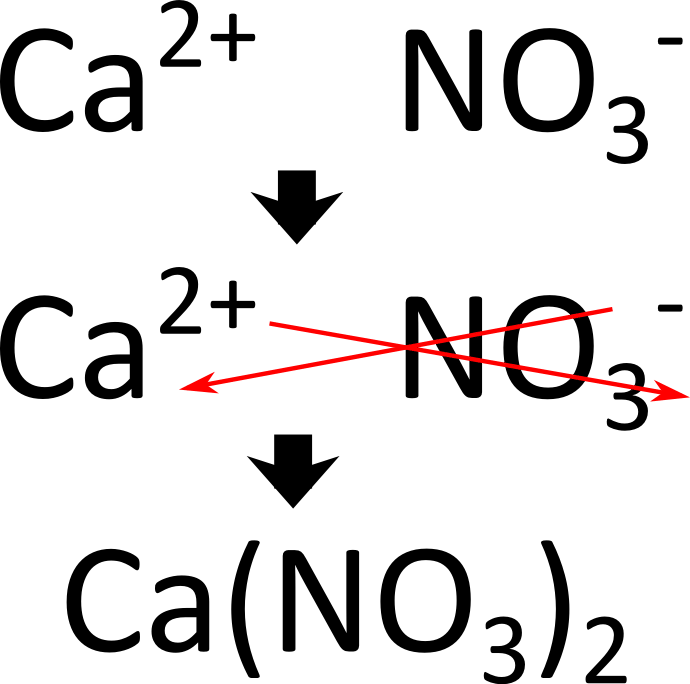 ?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?
?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?
 ?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?
?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?400
We call covalently bound groups of nonmetals which carry a charge, _____________.
What are polyatomic ions?
400
Carbon dioxide has two double covalent bonds. The total number of electrons shared between the single carbon atom and a single oxygen atom
What is four electrons?
The carbon atom shares 2 of its v electrons with one of the oxygen atom (which shares 2 of its v electrons).
The carbon atoms shares the other 2 v electrons it has with the second oxygen atom (which shares 2 of its v electrons). (a double bond means 2 shared pairs or 4 shared electrons. two double bonds means 2 sets of double bonds)
Bonus question:
How many total electrons are shared in this compound?
500
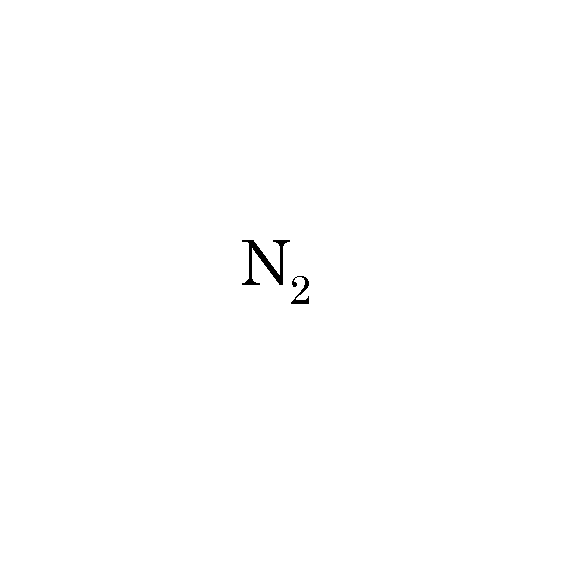
What is nitrogen?
Bonus question:
what type of covalent bond does this diatomic molecule have?
Be certain you can determine the difference between
a binary ionic compound and a diatomic molecule. The difference between a binary ionic compound and a ternary ionic compound. The difference between polar and nonpolar covalent compounds. The difference between ionic and covalent compounds.
500
Amonium Nitrate ( ) is an example of a(n) _________ compound.
) is an example of a(n) _________ compound.
 ) is an example of a(n) _________ compound.
) is an example of a(n) _________ compound.What is an ionic compound?
Make certain you check your list of polyatomic ions,  NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?
NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?
 NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?
NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?500
A nonmetal or nonmetal group bound to a metal makes up this type of compound.
What is an ionic compound?
Bonus question:
What do we call a neutral compound made up of multiple non-metals?
Bonus/Bonus question:
What group(s) of elements do not tend to bond with anything?
Bonus/Bonus/ Bonus question:
What group of elements does not participate in either ionic or covalent bonding but MAY indeed participate in some other form of bonding based on their position in the periodic table?
500
The symbols delta + and delta - mean


What is slight positive charge and slight negative charge respectively?
Bonus question:
Does this mean that the molecule has a difference in the total number of electrons vs the total number of protons? if not what does it mean? and what term do we use to describe the molecule?