When one chemical substance is transformed into one or more different substances
Chemical change
Convert 1,207 mm->m
1.207 m
What is the mass of an atom with 8 electrons, 8 protons, and 10 neutrons.
18 amu
A change that alters the form substance but not its chemical composition
Physical change
Convert 12.60 mL --> L
0.0126 L
Weighted average of the individual masses of the atoms that make up an element. (atomic weight shown in periodic table)
Average atomic mass
How many significant figures is in the number: 0.006070
4 sig figs
Atoms of the same element with different numbers of neutrons.
Isotope
Based on this diagram, which isotope of copper is least abundant?
Copper-65
Write in scientific notation with 4 significant figures:
12,785,000
1.279 x 107
How many neutrons is in: 136C
7 neutrons
Calculate the average atomic mass of Boron. (4 sig figs)

10.83 amu
Calculate the mass percent of hydrogen in hydroxide (OH) if hydrogen has a mass of 2.0 g and the total mass of OH is 17.0 g. (3 sig figs)
11.8% of H in OH
How many neutrons is in Chlorine-37
20 neutrons
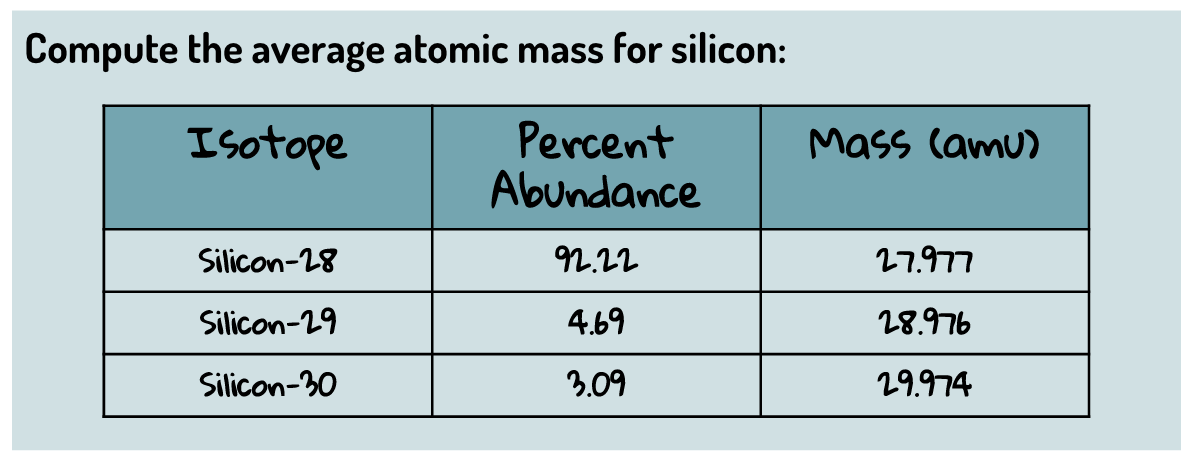
(4 sig figs)
28.08 amu