Isotopes have the same ____ but different ___.
atomic number (number of protons), mass number (number of protons + neutrons)
What does 14 stand for in the isotope carbon-14?
Mass number
The nuclear symbol for aluminum with a mass of 27.
1327Al
Copper 63 69.17%
Copper 65 30.83%
63.546 amu
Calcium has six isotopes Ca-40, Ca-42, Ca-43, Ca-44, Ca-46 and Ca-48. Given an average atomic mass of 40.078, which isotope of Calcium has the highest percent abundance?
Ca-40
True or False... Isotopes of an element have different number of neutrons.
True
What is the hyphenated symbol of this isotope?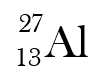
Aluminum-27 or Al-27
Write the nuclear symbol for the isotope with P=11, N=12.
2311Na
Eu-151 47.81%
Eu-153 52.19%
151.96 amu
Which is the most abundant isotope of helium?
He-3 or He-4
He-4
Why are Carbon-12 and Carbon-14 isotopes?
They have the same atomic number (number of protons).
The number of neutrons in silver-108.
61
Write the nuclear symbol for lead-208.
82208Pb
N-14 99.632%
N-15 0.368%
14.007 amu
What is the most abundant isotope of oxygen?
oxygen-16
oxygen-17
oxygen-18
oxygen-16
Why are these two atoms not isotopes?
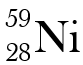
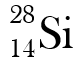
They have different atomic numbers.
A magnesium atom has 12 protons and 15 neutrons
Magnesium-27
The nuclear symbol for an isotope with N=82 P=60.
14260Nd
lead-204 1.4%
lead-206 24.1%
lead-207 22.1%
lead-208 52.4%
207.2 amu
Which isotope is the most abundant?
Re-187
Re-185
Re-187
True or False... Atom A has 13 protons and 14 neutrons. Atom B has 14 protons and 13 neutrons.
Atom A and Atom B are isotopes.
False. Isotopes have the same number of protons.
The hyphen notation name for an isotope with 46 electrons and 61 neutrons.
palladium-107?
Write the nuclear symbol for an isotope with N=125 and E=84.
20984Po
Uranium-234 0.0055%
Uranium-235 0.7200%
Uranium-238 99.2745%
238.03 amu
Which is the most abundant isotope of selenium?
Se-74
Se-76
Se-77
Se-78
Se-82
Se-78