What is the electron configuration for Li?
What is 1s2 2s1
What is the electron configuration of Cl?
What is [Ne] 3s2 3p5
What differs between isotopes of the same element
What is the number of neutrons?
In order to fill the 3d orbital, what orbital must be filled in first?
What is the 4s orbital
The part of an atom that has a positive charge?
What is the proton?
What is the Effective Nuclear Charge?
The net-positive charge experience by the nucleus
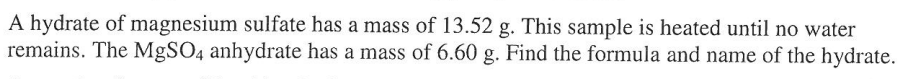
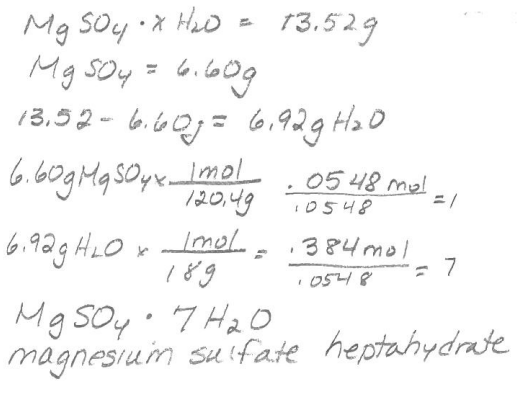
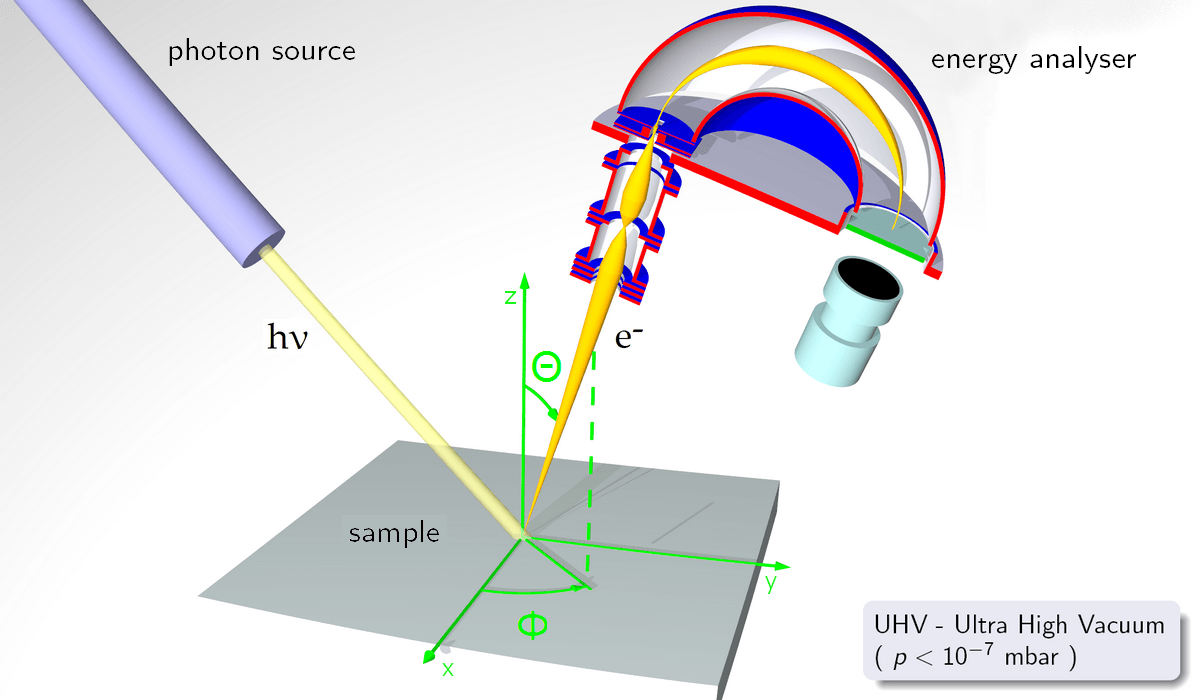
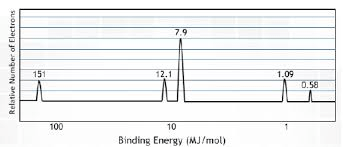
PES

Atomic radii of Li = 1.34 Å and Na = 1.54 Å
The valence electron for Li is in the 2s subshell, which is closer to its nucleus than the valence electron for Na, which is in the 3s sublevel.
What two words were combined to make the word ‘Spam’?
Spiced Ham

Will Ferrell
What is the electron configuration of F?
What is 1s2 2s2 2p5
What is the electron configuration of Ca?
What is [Ar] 4s2
The number that always stays the same for isotopes of same element.
What is the number of protons.
Where are valence electrons held?
In S and P in the highest energy level.
The two sub-atomic particles that make up the nucleus.
What are the protons and neutrons?
How many molecules are in 45.0 grams CH4?
What is Ionization Energy?
Energy Required to Remove and Electron

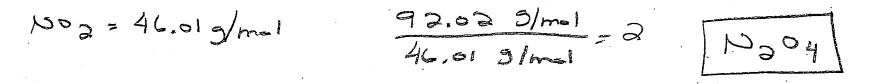
Atomic radii of Al = 1.18 Å and Si = 1.11 Å.
Si has 4 unshielded protons (protons – core electrons), which attract the valence electrons closer to the nucleus than the 3 unshielded protons in Al.
What are the names of the Rice Krispies mascots?
Snap, Crackle, & Pop

Amy Poehler
What is the electron configuration of Al3+?
What is 1s2 2s2 2p6
What is the electron configuration of Zn?
What is [Ar] 4s2 3d10
How are the following three atoms related: C-11, C-12 and C-13
What is all three isotopes of carbon?
True or False
The orbitals are filled from highest energy level to the lowest energy level.
What is false?
237.98 amu
What is Coulombs Law?

Increase or Decrease
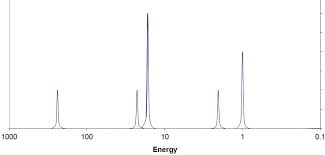
First ionization energies for B = 801 kJ/mol and Al = 578 kJ/mol.
The ionized electron for B comes from the second energy level, which is at a lower energy state than the ionized electron from Al, which comes from the third energy level ∴ it takes more energy to ionize.
What is gelatin made from?
The skin and bones of animals

Jason Sedakis
What is the electron configuration of Ti+2?
What is 1s2 2s2 2p6 3s2 3p6 3d2
What is the electron configuration of Ag?
What is [Kr] 5s2 4d9
How many neutrons does Gold-197 have?
What is 118 Neutrons?
True or False
Two electrons can occupy a single orbital if they have the same spin.
False
These two numbers are ALWAYS equal and never change.
What is atomic number and number of protons?
How many total atoms are in 5.8 mol CaCl2?
Calculate the empirical and molecular formulas of adrenaline. The compound has a molecular mass of 183 g/mol, and is 59.0% C, 7.1% H, 26.2% O, and 7.7% N by mass.
C9H13O3N
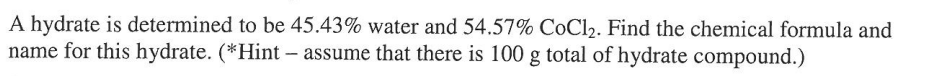
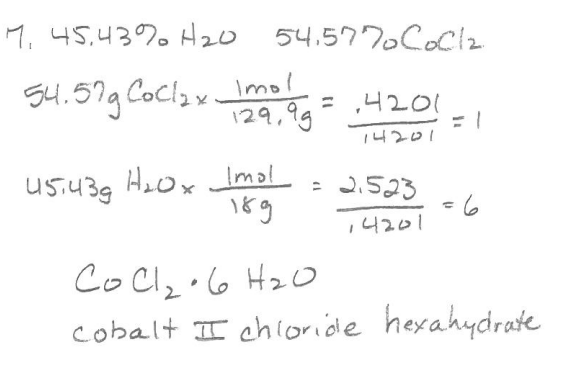
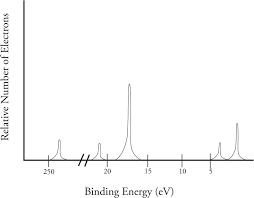
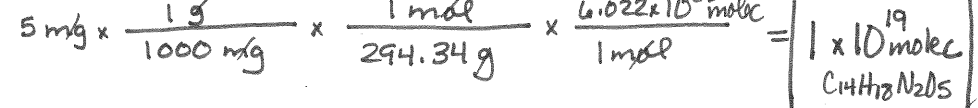
For Na, the first ionization energy I1 = 495 kJ/mol and second ionization energy I2 = 4562 kJ/mol
I2 is much larger than I1 because the second ionized electron comes from the core rather than valence shell.
What cheese would you find on a Reuben sandwich?
Swiss cheese

Kate McKinnon
What is the electron configuration of Ni?
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d8
What is the electron configuration of Ba?
What is [Xe] 6s2
The mass number of a lead isotope with 125 neutrons
What is 207?
True or False
Electrons in 1s would have a lower binding energy compared to electrons in 2s?
False
The part of the atom that contains the energy levels around the nucleus.
What is the electron cloud?
How many total ions are in 2.95 grams MgSO3?
Nicotine is 74.1% C, 8.6% H, and 17.3% N by mass. The molecular mass of nicotine is 162 g/mol. Calculate its empirical and molecular formulas.
C10H14N2
Why Nitrogen is smaller than Be.
N experiences an effective nuclear charge of +5 (Zeff=7-2=5), Be Zeff= +2. The valence e- of N are “feeling” the pull of 5 protons as opposed to 2 e- for Be
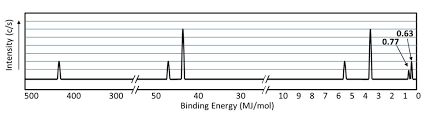
The electron affinities for Mg > 0 and Na = -53 kJ/mol.
The added electron to Mg must go into a higher energy state (3p) compared Na (3s) ∴ the loss in energy when an electron approaches a nucleus is offset by the electron's higher energy level.
How many herbs and spices make up the KFC spice blend?
11

Kristen Wiig