22.4 L
What is the volume occupied by one mole of a gas at STP
Earth is this system.
What is defined as a closed system where it is part of the universe surrounded by a boundary, where no exchange of matter can take place?
Oscillating electric and magnetic fields
What are electromagnetic waves.
A matter wave
What is possible only in specific energy levels?
Integers coming from solutions to wave equations that describe specific properties of electrons in atoms.
What are quantum numbers
The total pressure inside a cylinder containing 2 mol of O2 and 2 mol of N2
What is the sum of their partial pressures.
Releases energy as heat
What is an exothermic process.
The black body radiation is explained by Rayleigh - Jean's equation
What is when the wavelength approaches zero, the intensity of the radiation approaches infinity.
The energy emitted by electrons when it moves from one energy state to the other.
What are the quanta of energy?
There are 9 total orbitals.
What is when principal QN = 3
The internal energy of monoatomic gases
What is equal to its Translational Kinetic Energy NKEave.
when q and work change.
What is the change in the internal energy U of a system?
What is the product of the frequency of light and Planks' constant?
The Balmer series emissions happen
What is the movement of electrons in one electron systems?
Which one of these are allowed?
a)n=3, l=2, ml=0
b)n=1, l=1, ml=-1
c)n=0, l=0, ml= 0
d)n=5, l=1, ml=6
What is a)?
Variation of average velocities with molar mass :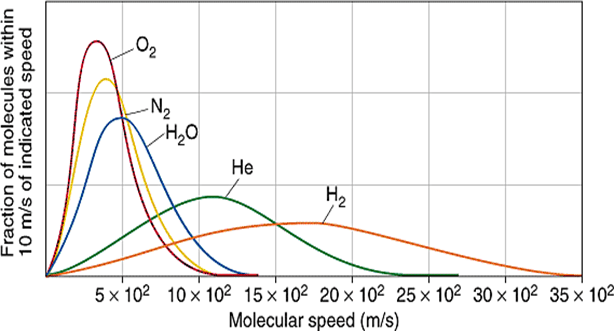
What is the implication of root mean square velocity?
I can measure the change in the heat energy of the system.
What is the change in Enthalpy?
When a polarized light is incident on a metal, it can eject electrons from its surface immediately.
What is the photoelectric effect.
The shortest wavelength emitted when the nf ==> infinity.
1/𝜆= 𝑅𝐻(1/𝑛𝑓^2 −1/𝑛_𝑖^2 ); Rydberg’s const = 1.09737x10-7 m-1
What is 364.6 nm?
The temperature of this room (22 0C) right now in Kelvin
What is 22 C+ 273.15 =295.15K?
Mean free path
What is increases with decreasing pressure and decreases with increasing pressure?
This molecule, essential for life has a very high specific heat capacity
What is the specific heat capacity of water?
Each atom in an element absorbs and emits only these light waves.
What are the spectral lines.
The wavelength is an integral multiple of the wavelength when the electron is in this
lambda
What is a Bohr's circular orbit?
A lab technique used to measure the amount of heat transferred to or from a substance.
What is calorimetry?