These are the substances that exist before a chemical change
Reactants
These are the new substances formed as a result of a chemical change
Products
What is an ion?
An atoms or group of atoms that has a positive or negative charge
(different number of electrons compared to protons)
What is a covalent bond?
A chemical bond that forms because of shared electrons
In the following chemical formula, which number is the subscript?
3CH4
4
As opposed to a molecule that is overall neutrally charged, water molecules have both slight negative and slight positive charges. This makes water what type of molecule?
Polar molecule

This was the word of the week from Matins
Sanctification
What are homonuclear diatomic molecules?
Two atoms of the same element bonded together (example: O2)
This is the number listed before an atom or molecule in a chemical equation (indicates quantity)
Coefficient
Are cations positive or negative?
Positive
(Think: ca+ion)
What holds atoms together in a covalent bond?
Shared electrons travel around both nuclei
In the following chemical formula, which number is the coefficient?
3CH4
3
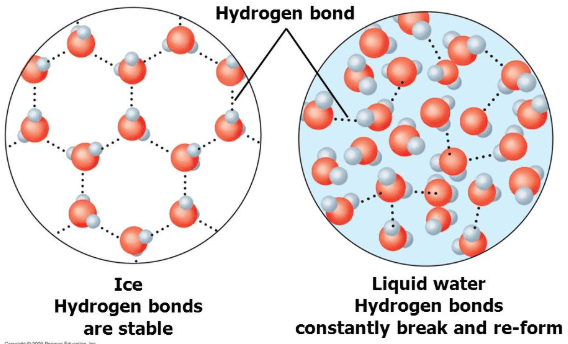
This is the attractive force between water molecules (not atoms)
Hydrogen bonds
This person taught most of the chapel messages on the book of James
Mr. Bisulca
Atoms are most stable when this is full.
Valence shell/
Outermost electron shell/
Highest energy level electron shell
How does forming or breaking chemical bonds affect the number of protons in an atom?
The number of protons does not change
Are anions position of negative?
Negative
What kind of bond is represented in this structural formula?
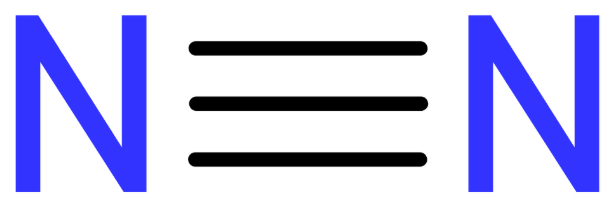
Triple covalent bond
In the following chemical formula, how many carbon atoms are represented?
3CH4
3
What feature of water's chemical structure causes it to have a relatively high boiling point?
Hydrogen bonds
Based on this week's catechism question, what does God require in the second commandment?
That we avoid all idolatry and do not worship God improperly
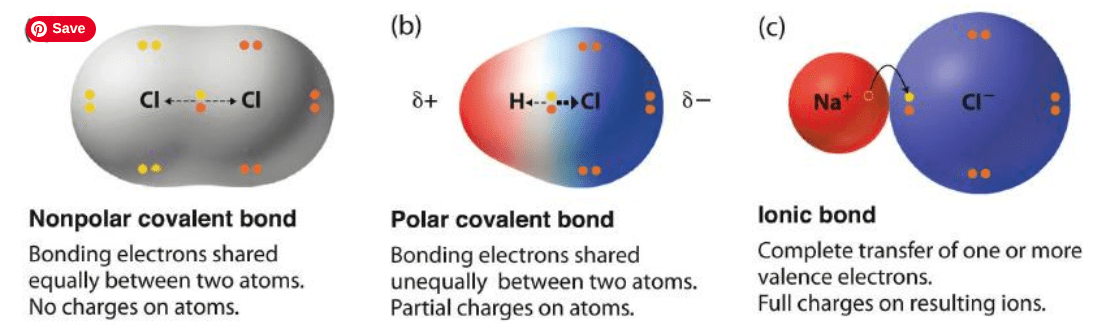
This is a force of attraction between atoms or a group of atoms due to the sharing or transferring of electrons
Chemical bond
What is the name for this kind of diagram?
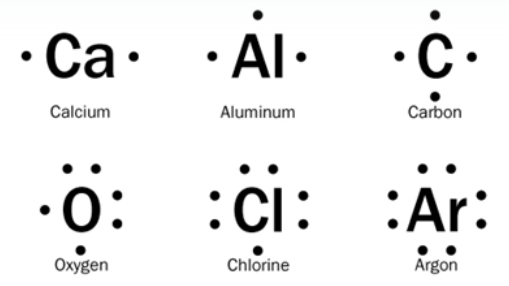
Electron dot diagram
In ionic bonds, one atom ____ its electron(s)
Transfers, gives, loses, receives, gains
This is a covalent bond where one atom has a stronger pull on the shared electron(s) than the other
Polar covalent bond
In the following chemical formula, how many hydrogen atoms are represented?
3CH4
12
This property of water causes water molecules to attract and stick to other water molecules
Cohesion
This person was the first woman to earn a medical degree (from Geneva college)
Elizabeth Blackwell
What is the name for this type of atomic model?
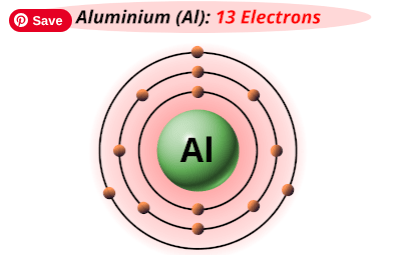
Bohr model or planetary model
Fill in the blank in this chemical equation:
_NO2 ---> 2NO + O2
2
What holds atoms together in an ionic bond?
Oppositely charged ions are attracted to one another
What types of elements usually form covalent bonds?
Non-metals
What kind of covalent bonds are shown in this model of methane?
Single covalent bonds
This property of water causes water molecules to attract and stick to other substances
Adhesion
This person is credited with being the first person to use general anesthesia
Dr. Horace Wells