This is a positively charged particle
What is a proton?
These electrons are located on the outermost electron shell, and are the one that matters most!
What are valence electrons?
This is the picture for Hydrogen.

This is a type of covalent bond where atoms share the electrons equally (atoms have equal pull)
What is nonploar covalent bond?
An atom or a group of atoms that has an electric charge
What is an ion?
This is a negatively charged particle.
What are electrons?
How many electrons are held in shells 1, 2, and 3?
What is 2, 8, and 8?
This is the picture for Lithium.

In a double bond, this many electrons are shared.
What is 4?
this type of ion is formed when an atom loses an electron.
What is a positive ion?
These 2 subatomic particles make up the nucleus of an atom.
What are protons and neutrons?
This number tells you how many protons an atom has.
What is atomic number?
This is the picture for carbon.

A molecule in which the polar covalent bonds to not cancel out will be this.
What is a polar covalent bond?
These 2 types of ions are attracted to one another.
What are positively charged and negatively charged ions?
Adding/taking this from an element will make it something completely different.
What are protons?
This rule says that atoms "want" 8 electrons in their valence shell.
What is the Octet rule?
Draw an example of a double covalent bond!
Use the lecture powerpoint!!!
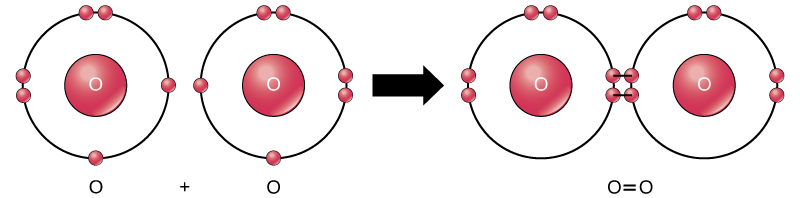
A neutral group of atoms joined by covalent bonds is called this.
What is a molecule?
Ions are formed when atoms lose or gain this...
What is a valence electron?
What are Little Boys and Girls REALLY made of!?
What is "CHON"?
Carbon, Hydrogen, Oxygen, and Nitrogen
How many electrons are in hydrogen gas?
Draw on the board if you need to!
What is 2?
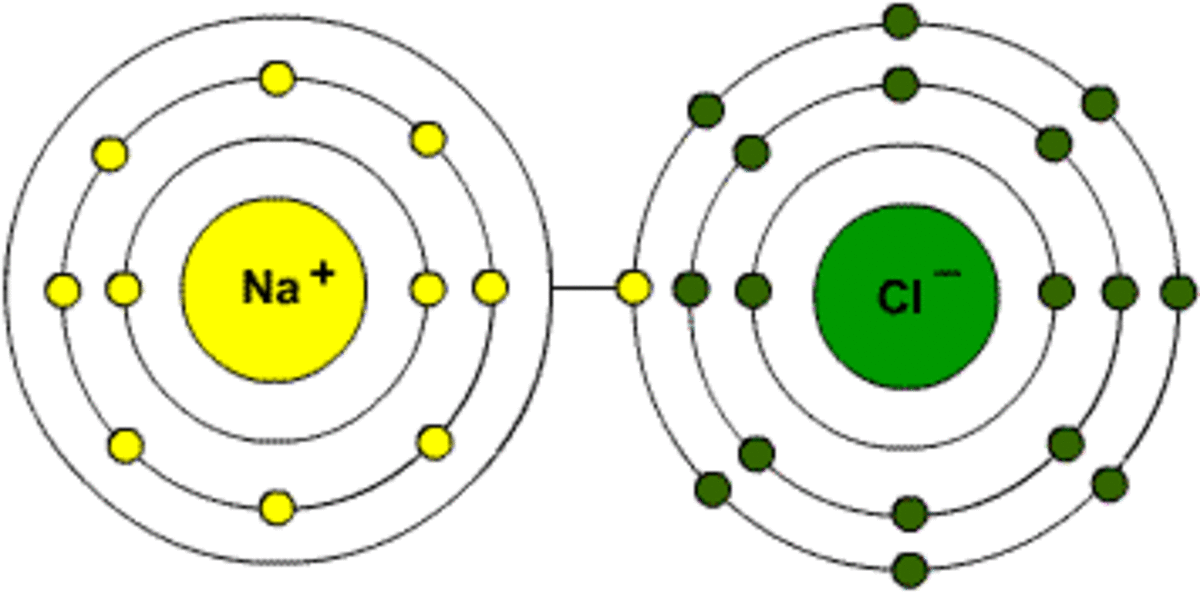
This is the picture for NaCl
A chemical bond formed when atoms share three pairs of electrons.
What is a triple bond?