True or False
An acid with a higher pka value is more acidic.
False
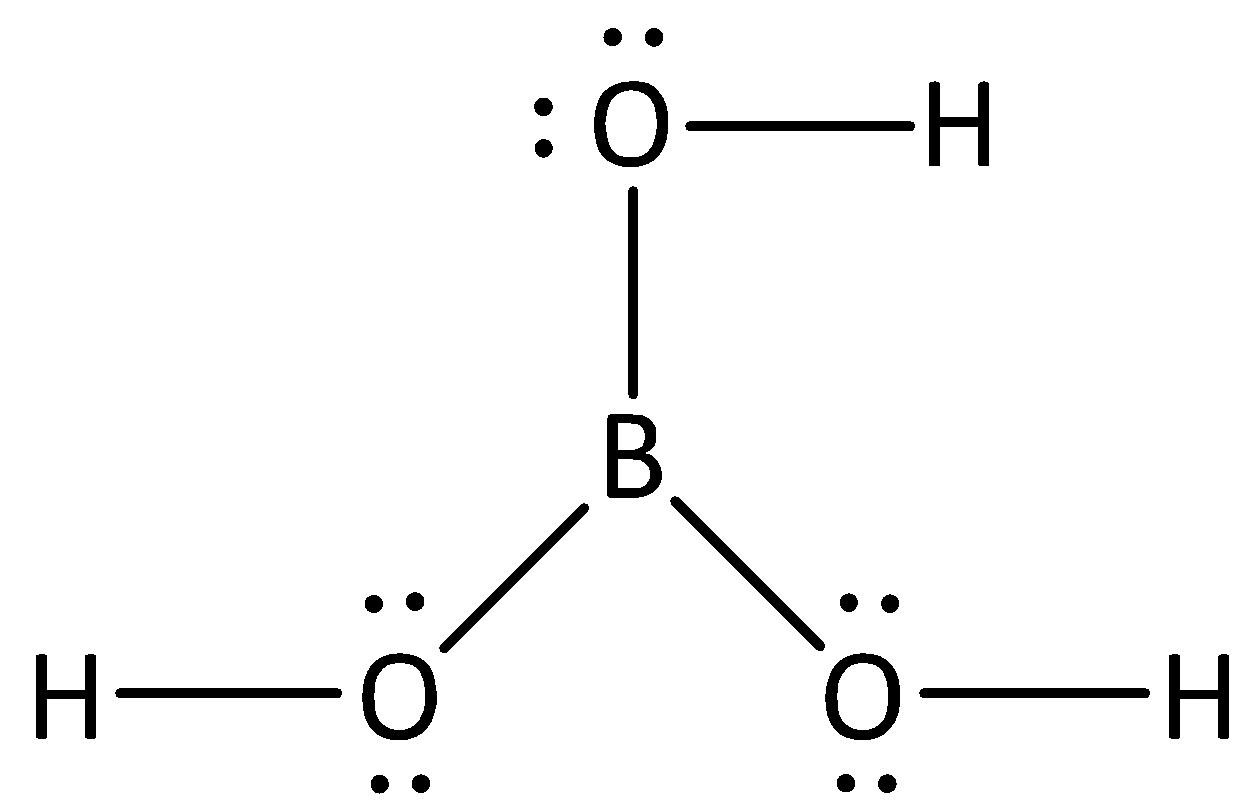
Is this image a base?
What is the percentage of s character in an sp^3 orbital?
25%
True or False
A more stable conjugate acid means the original acid was more reactive.
True
True or False
Atoms in the same column will stabilize negative charges equally.
False, larger atoms stabilize negative charges better.
Would a lone pair be more stable if it were in an sp^3 orbital or an sp orbital?
sp
Is a Lewis acid an electron pair donor?
 What charge would ammonia have if it reacted with an acid?
What charge would ammonia have if it reacted with an acid?
Positive
 what is the hybridization and Formal charge of this carbon?
what is the hybridization and Formal charge of this carbon?
sp2
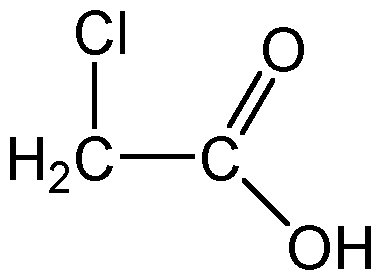
This is Chloroacetic acid. Would you expect it to be more or less acidic if the Chlorine was a fluorine atom?
Would an sp carbon or sp3 carbon be more basic?
sp3
Does sp4 exist?
No, only 3 p orbitals are available for hybridization.
Draw the electron pushing arrows for the following acid-base reactions and determine which side the equilibrium lies.

Draw on board
Which is a stronger base?
OH^-1 or SH^-1
OH^-1
draw what happens when you blend an s and p orbital.
draw on board