What is meant by the rate of a chemical reaction?
The time taken for products to get made or reactants to get used up
Which term describes a molecule that contains two or more elements chemically combined in fixed proportions? 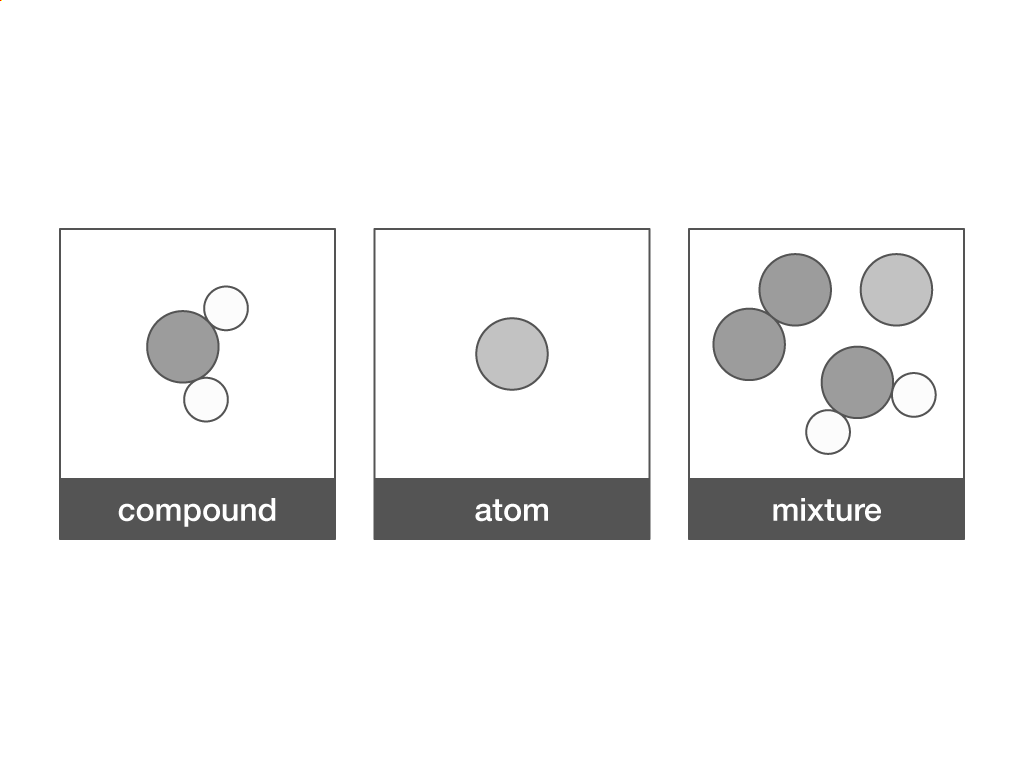
Compound
Chemical bonding happens because all atoms are more stable if they have a ______ outer shell
full
What kind of reaction releases energy to the surroundings?
Exothermic
What are the four factors that affect the rate of reaction?
1. Temperature
2. Concentration/pressure
3. Surface area
4. Presence of a catalyst
Distillation can be used to separate substances in a solution due to a difference in their ________ points.
boiling
Metals ________ electrons, while non-metals ___________ electrons
lose, gain
What kind of reaction causes a temperature increase?
Exothermic
Calculate the mean rate at 40 degrees C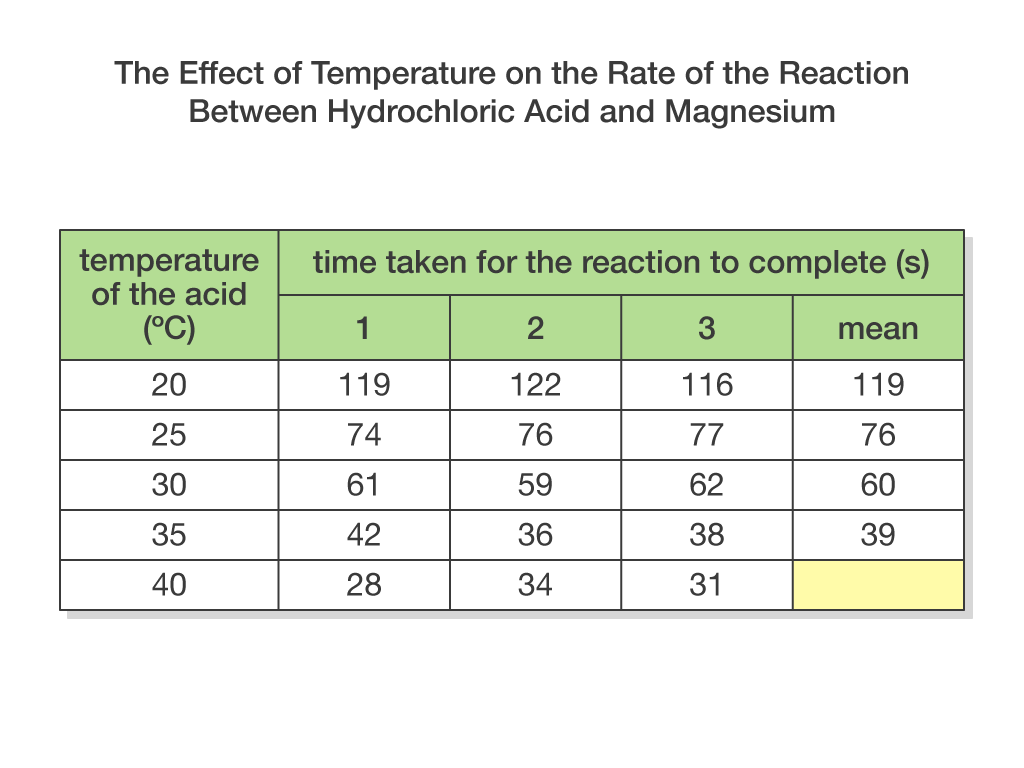
31
What is the symbol for hydrogen?
H
What charge does a sodium ion have? Sodium is a metal in group 1
+1
What is the missing label for this energy profile diagram?
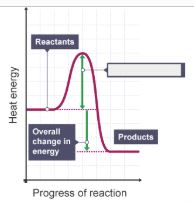
Activation energy
Explain how surface area affects the rate of reaction
The greater the surface area, the more particles are available to react at a given time. This leads to more frequent successful collisions
2 electrons on the first shell; 8 electrons on the second shell; 8 electrons on the third shell
-1
Describe what is meant by activation energy
The minimum energy with which particles need to collide to cause a reaction
Why might the apparatus shown result in inaccurate readings for the volume of gas produced?
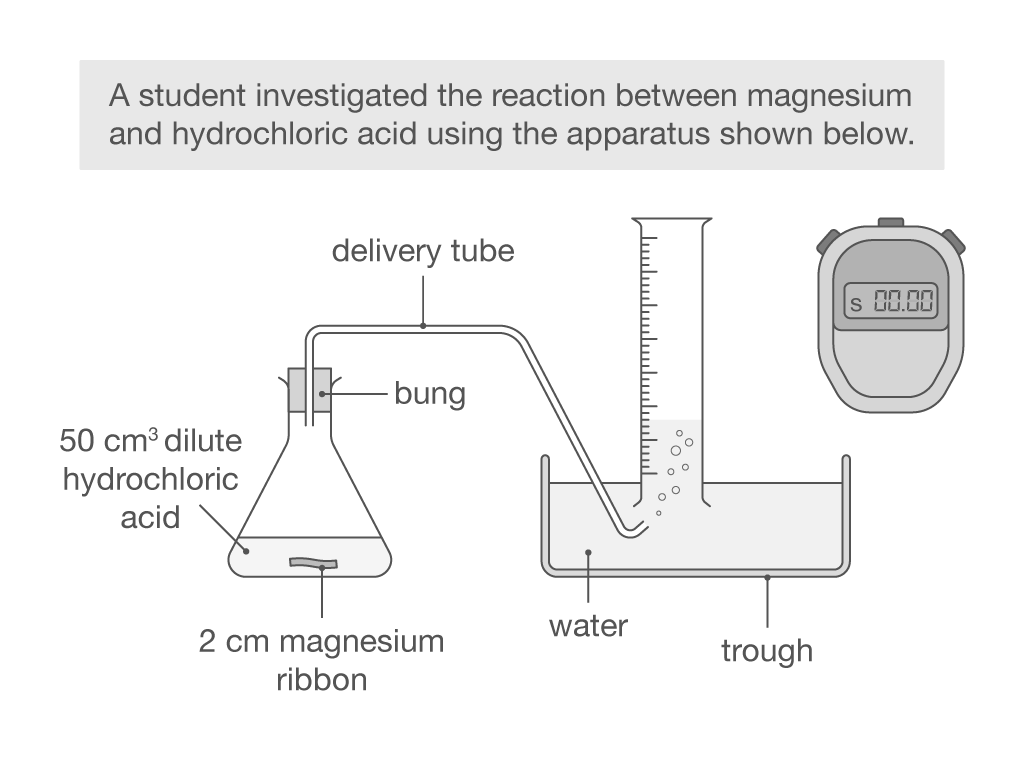
Delivery tube is not directly in the measuring cylinder, so some gas will not be collected
How many protons are in an atom of calcium?
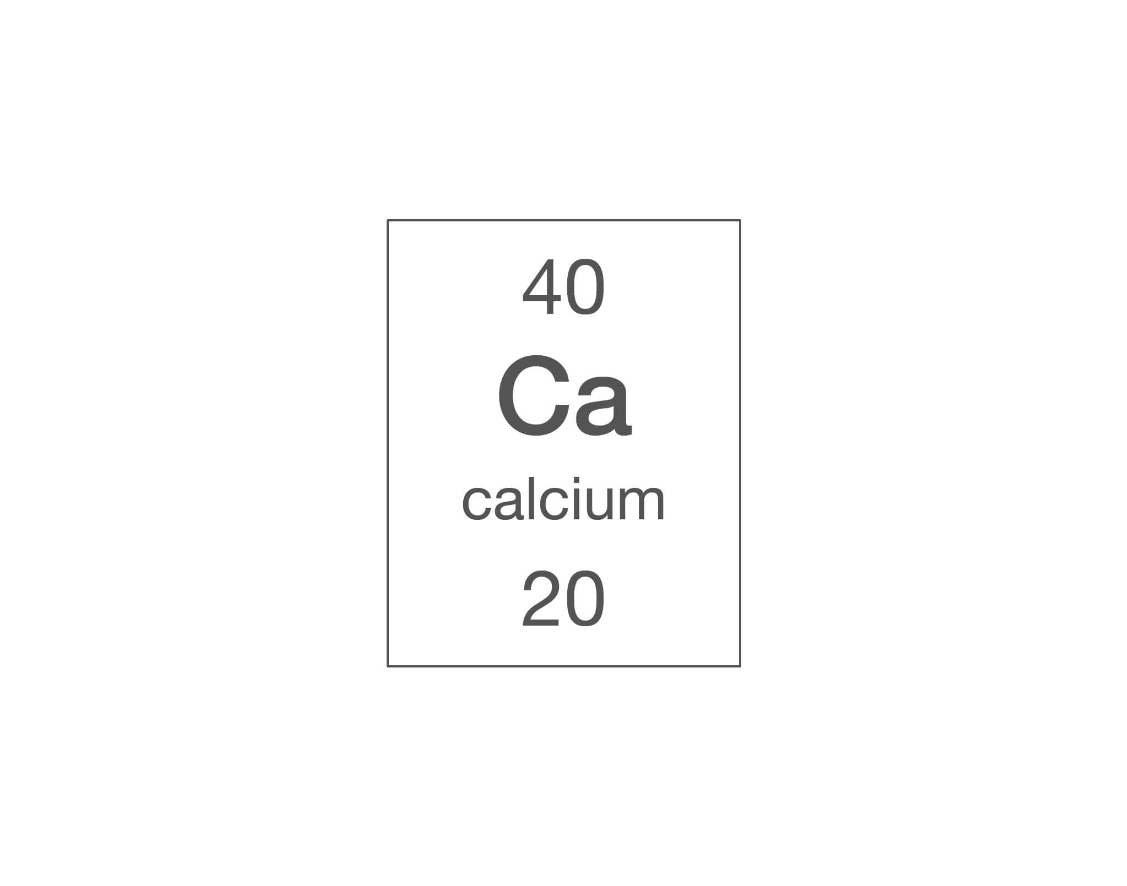
20
What is the formula of sodium fluoride? Sodium ions have a charge of +1, while fluoride has a charge of -1
NaF
What is the independent variable in this experiment?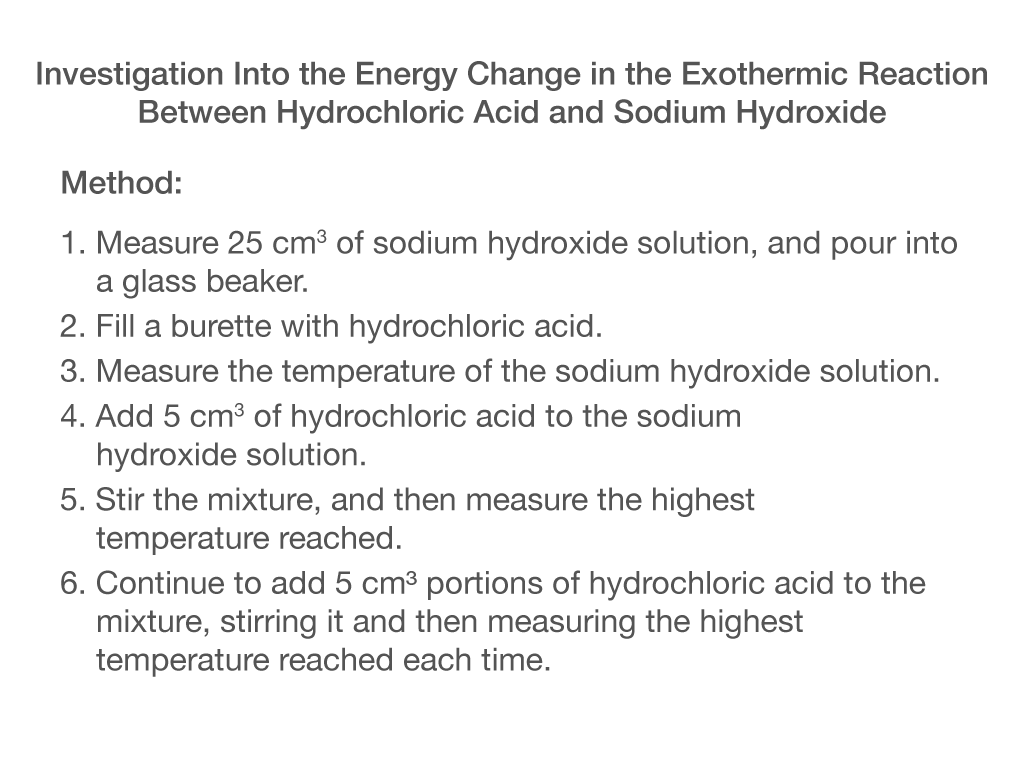
Volume of hydrochloric acid