How many electrons can the first ring/orbital shell hold?
2
What does the atomic number of an element represent?
number of protons found in that atom
On a periodic table, what are rows called?
periods
An atom’s mass number is 210 and its atomic number is 85. How many neutrons does the atom have?
125
What element is in the 4th period and 12th group?
zinc
What main group of elements have solids, liquids and gases at room temperature?
non-metals
The ability to be SHAPED/BENT into different shapes without breaking:
What is malleability?
What subatomic particles make up the mass of an atom?
protons and neutrons
What do all elements in the same row have in common?
Same number of shells
Would metals, nonmetals, or metalloids be described as having the following properties?
luster, malleable, ductile, good conductors, donate electrons, high density, high melting point
metals
What element was named for the creator of the periodic table?
101 - Mendelevium
Which noble gas is an exception to the group?
Helium
What is the name of the 3 main types of elements on the periodic table?
metals, non-metals, metalloids
The staircase follows what group of elements down the periodic table?
metalloids
What is the name of the vertical organization of the elements on the periodic table?
group or family
What are three physical properties of non-metals?
not shiny, not malleable (brittle), not ductile, poor conductors (good insulators), gain electrons, low density, low melting point
The element is located at the 6th period and 2nd group?
What is Barium?
The ability to be made into a wire:
ductility
The number of valence electrons in group 15.
What is 5?
Which groups house the transition metals?
Groups 3-13
What do all the elements in the same column have in common?
same number of valence electrons
What element is grouped with alkali metals (Group 1) even though it is not an alkali metal.
Hydrogen
What is the first element in period 3?
Sodium
What is the name of the group 2 family?
alkaline earth metals
The majority of the elements on the periodic table are classified as this:
metals
Argon belongs to what specially named group?
noble gases
Which family (name, not number) is the most reactive metals family with one valence electron?
Alkali Metals
What is the name of group 17?
Halogens
How many electrons does a neutral atom of iodine have?
53
What category does the image represent?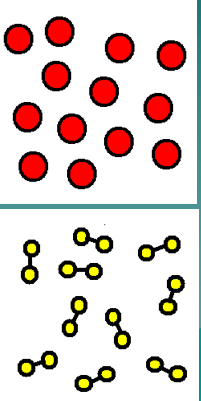
Elements
What Periodic Element symbol has an Atomic Mass (Weight) of 92.91
Nb
Where does bonding occur between atoms?
At the valence electron shell/orbital.
Who is the creator of the Modern Periodic Table?
Who is Dmitri Mendeleev
An atom contains 14 protons, 17 neutrons, and 12 electrons. What is the atomic charge?
What is +2?
A Carbon atom has 8 electrons. What is its charge?
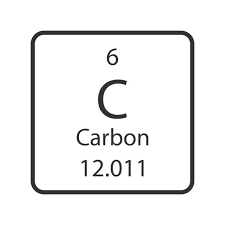
What is -2?
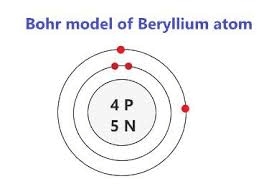
Draw the Bohr Model for Beryllium.