Which vocabulary word describes: an individual particle of an element
Atom
How many different types of atoms occur naturally on earth?
92
How many elements naturally occur on earth?
92
What direction are Periods?
Horizontal (left to right)
List 1 element (chemical symbol) that will have similar properties to calcium (Ca) #20
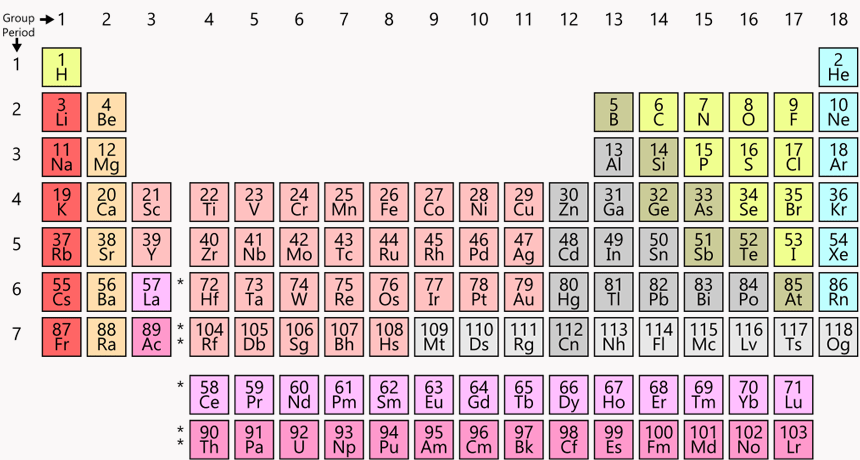
Be, Mg, Sr, Ba, or Ra
Which vocabulary word describes: a unique particle of matter that is a fundamental building block of matter
Element
What unit is used to measure the size (length) of an atom?
Picometer
What is Carbon's atomic number?

6
What direction are groups?
Vertical (up and down)
List 1 element (chemical symbol) that will have similar properties to carbon (C) #6
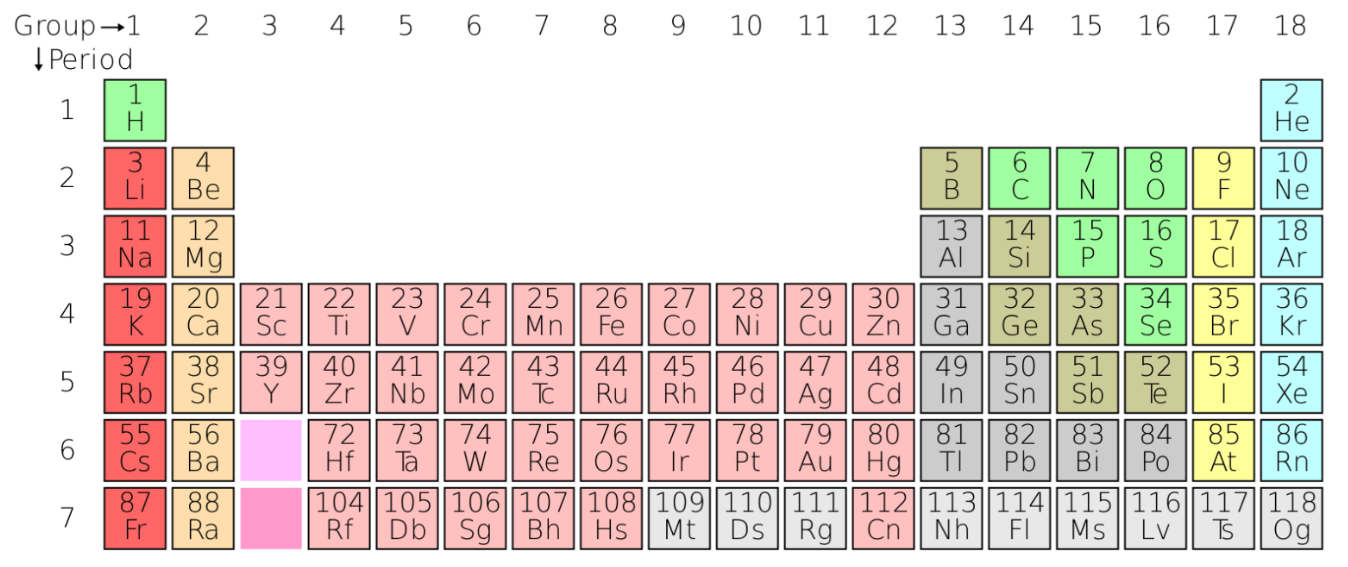
Si, Ge, Sn, Pb or Fl
Which vocabulary word describes: the mass of an atom; all atoms of an element have the same atomic mass
Atomic mass
What unit is used to measure the mass of an atom?
Daltons
How many protons does Carbon have?

6
Elements in the same _____________ tend to have similar properties.
Group / Family/ Column
What period and group is xenon (Xe) #54
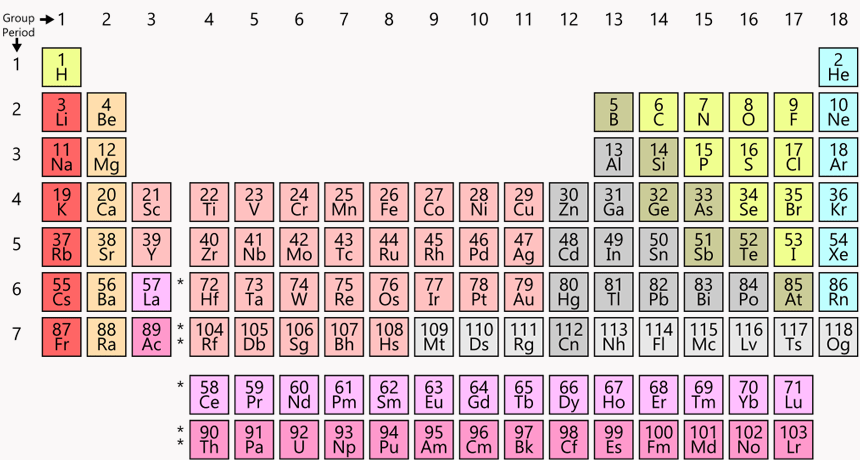
Period 5
Group 18
Which vocabulary word describes: a shorthand notation of one, two or three letters that represent the name of the element.
Chemical symbol
What is the scale used to measure the size of atoms?
Atomic scale
How are elements organized in periods across the periodic table?
By increasing mass, protons and atomic number
Who created today's current version of the periodic table?
Dmitri Mendeleev
What period and group is nitrogen (N) #7
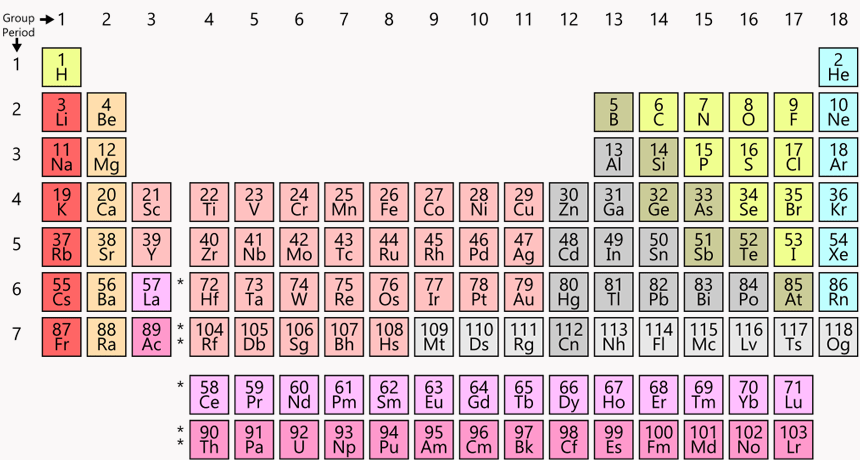
Period 2
Group 15
Which vocabulary word describes: a table that lists all of the known elements and groups them into columns by important shared properties
The Periodic Table (of Elements)
Convert 0.000000000058 meters (the size of a neon atom) into scientific notation.
5.8 x 10-11
How are elements organized in groups/ families?
- Same number of valence electrons
- Similar properties
How did Dmitri Mendeleev organize the elements in the periodic table?
1.) Periods/ rows based on their atomic mass/ number/ protons
2.) Groups based on their properties
What period and group is potassium (K) #19
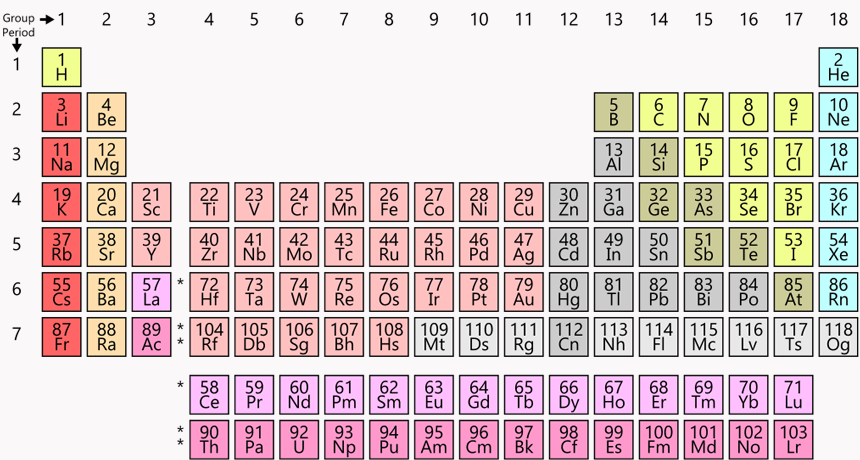
Period 4
Group 1