This states that matter cannot be created nor destroyed.
What is Law of Conservation of Mass?
This is found on the left side of the yields sign. In other words, what goes in to the reaction.
What is reactant?
The first step, other than making a REP table, that you do when balancing a chemical equation.
What is count atoms.
This type of reaction only produces one product.
What is a synthesis reaction?
Forms between a metal and a nonmetal.
What is an ionic bond?
By definition, the Law of Conservation of Mass states that matter (mass) may not be __________ nor _________ .
What is created /destroyed?
This sign indicates the direction of the reaction, and separates reactants from products.
What is yield or arrow?
Tells you the number of molecules of a given substance in a chemical equation.
What is coefficient?
This type of reaction only has one reactant, but multiple products.
What is decomposition?
A metal that loses an electron forms this type of ion.
What is a cation?
The masses of the reactants and the products in a chemical reaction according to the Law of conservation of mass should always be ____.
What is equal?
It is the end result of a chemical reaction.
What is product?
Tells you if the equation if balanced.
What is the number of atoms in the product side is equal to the reactants?
The type of reaction shown below:
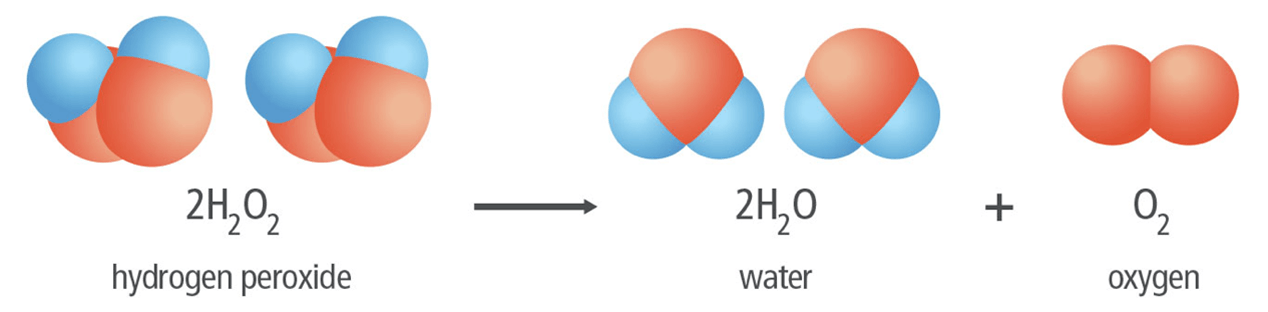
What is decomposition?
Forms between two nonmetals.
What is a covalent bond?
Twelve grams of reactant X react with fifteen grams of reactant Y. This is the total mass of the product(s).
What is twenty seven (27) grams?
This tells the number of atoms in a chemical equation.
What is subscript?
The number of elements in this equation:
CaCl2 → Ca + Cl2
What is two (2)?
The type of chemical reaction shown by this equation: CaCl2 + Cl → CaCl3
Synthesis
Type of bond that results in high melting and boiling points.
What is an ionic bond?
The type of reactions shown below.
What is a double replacement?
The balanced equation for:
CaCl2 → 2Ca + Cl2
What is
2CaCl2 → 2Ca + 2Cl2?
The type of chemical reaction shown below.
:max_bytes(150000):strip_icc()/single_displacement_reaction-56a1327a3df78cf7726851ad.png)
The chemical formula for table salt.
What is NaCl?