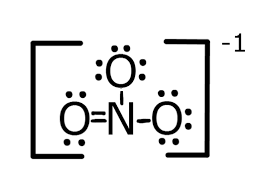
What is the Lewis structure of Nitrate?
What is the Lewis dot structure of Water?
Draw Lewis Dot of Sodium Hydroxide.
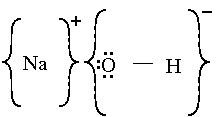
What is the HONC Rule?
H- 1 bond
O- 2 Bond
N- 3 Bond
C- 4 Bond
What is the Lewis structure of Ammonium?
What is the Lewis structure of CH4?

What is the Lewis structure of NaCl?

What element can never be the central atom?
Hydrogen
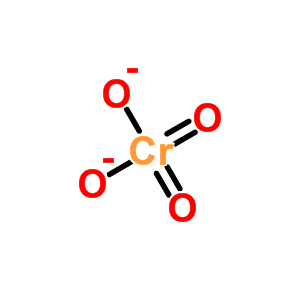
What is the Lewis structure of Chromate?
What is the Lewis structure of HF?

What is the Lewis structure of calcium carbonate?

What can never occur with electrons in a Lewis dot structure?
There can never be just one electron. There always has to be two
What is the Lewis structure of Sulfate?

What is the Lewis structure of Sulfur Dioxide?

What is the Lewis structure of Magnesium nitrate?
How do we know what the central atom is?
Based on which atom has the lowest electronegativity
What is the Lewis structure of Cyanide?
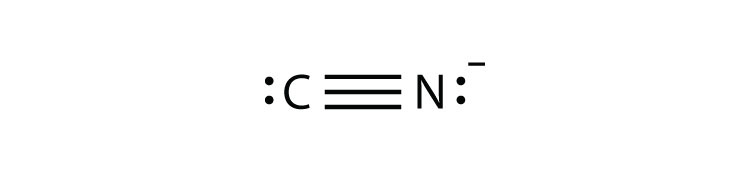
What is the Lewis structure of Carbon Dioxide?
What is the Lewis structure of Barium Nitrate?
When do we use double and triple bonds?
When the HONC rule tells us that we have to use it
