What is a lone pair?
Electrons that are not shared with other elements
A Lewis structure is polar when...
All the atoms touching the center are not the same
A stronger IMF will take _______ to melt
Longer
What is a bonded pair?
Electrons that are shared with other elements
What is the polarity of this Lewis Structure?
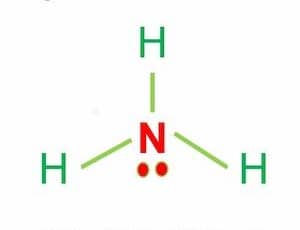
Polar
_____ is the only nonpolar IMF
LDF (London Dispersion Force)
IMF stands for...
Intramolecular Forces
H-bonds and Dipole-Dipole are...
Polar
What is the IMF of hydrogen fluoride?
H-Bond
H-Bonds are only between...
Nitrogen, oxygen, or fluorine
Draw NO3's Lewis structure and determine the polarity
Non-polar
What is the weakest IMF?
London Dispersion Force
VSEPR is...
The shape of the molecule
Draw Cl2's Lewis structure and determine the polarity
Non-polar
Put the IMF types in order from strongest to weakest.
H-bond, Dipole-Dipole, LDF